Search Results
Showing results 101 to 120 of 234

Condensation
Source Institutions
In this activity, learners explore the process of condensation.

Cartesian Diver
Source Institutions
In this demonstration, learners observe the effects of density and pressure. A "diver" constructed out of a piece of straw and Blu-Tack will bob inside a bottle filled with water.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.
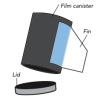
Pop Rockets
Source Institutions
In this activity, learners make film canister rocket ships. A fin pattern is glued onto the outside of the canister, and fuel (water and half an antacid tablet) is mixed inside the canister.

Exploring Products: Nano Fabrics
Source Institutions
In this activity, learners explore how the application of nano-sized "whiskers" can protect clothing from stains.

Temperature Affects Dissolving
Source Institutions
Learners design their own experiment to compare how well cocoa mix dissolves in cold and hot water. They will see that cocoa mix dissolves much better in hot water. Adult supervision recommended.
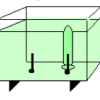
Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.
Snow Day!
Source Institutions
In this activity (on pages 4-5), learners make fake snow by adding water to the super-absorbant chemical from diapers, sodium polyacrylate.

Look-alike Liquids
Source Institutions
Learners add drops of four liquids (water, alcohol, salt water, and detergent solution) to different surfaces and observe the liquids' behavior.

M&M's in Different Temperatures
Source Institutions
Learners design their own experiment to investigate whether the temperature of the surrounding water affects the rate at which the colored coating dissolves from an M&M.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
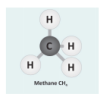
Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).
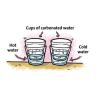
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.

A Pressing Engagement
Source Institutions
In this quick and easy activity and/or demonstration, learners illustrate the effect of the weight of air over our heads.

Which Powder is It?
Source Institutions
In this chemistry challenge, learners identify an unknown white powder by comparing it with common household powders.

Plant Power
Source Institutions
In this chemistry challenge, learners identify which plants have the enzyme "catalase" that breaks hydrogen peroxide into water and oxygen.
Egg Osmosis
Source Institutions
Visitors observe three beakers. One beaker contains an egg immersed in vinegar. Visitors observe carbon dioxide gas escaping from the shell as the calcium carbonate reacts with the vinegar.
