Search Results
Showing results 1 to 20 of 144

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Walk On Water Bugs
Source Institutions
In this activity (on pages 29-35), learners examine water pollution and filtration.

Water: Clearly Unique!
Source Institutions
In this activity on page 4 of the PDF (Water in Our World), learners conduct some quick and easy tests to determine the differences between water and other liquids that look very similar to water.
Enhanced Water Taste Test
Learners conduct a "blind" taste-test of several types of enhanced or fitness water drinking water that has commercially added substances like vitamins, sugars, or herbs.

Water Illusions: Refraction & Magnification
Source Institutions
Learners demonstrate how water can distort, refract and magnify light.

Breaking the Tension: Surface Tension 1
Source Institutions
Learners explore how the attractive forces between water molecules create surface tension and allow certain objects to float on the surface of water.

Comparing the Density of an Object to the Density of Water
Source Institutions
Learners compare the weight of equal volumes of wax, water, and clay. Learners discover that since the wax weighs less than an equal volume of water, it is less dense than water and will float.

Changing the Density of a Liquid: Heating and Cooling
Source Institutions
Learners investigate how the temperature of water affects its density.

Moving On Up: Capillary Action 1
Source Institutions
Over the course of several days, learners explore the property of water that helps plants move water from roots to leaves or gives paper towels the capacity to soak up water.

A Little Drop of Water: Cohesion
Source Institutions
Learners explore water's property of cohesion through two investigations.
Pepper Scatter
Source Institutions
In this activity, learners explore the forces at work in water. Learners experiment to find out what happens to pepper in water when they touch it with bar soap and liquid detergent.

Formation of a Precipitate
Source Institutions
Learners create hard water by mixing Epsom salt and water. Then they compare what happens when soap solution is mixed with hard water and regular water.
How Does Water Climb a Tree?
Source Institutions
In this activity, learners conduct an experiment to explore how water flows up from a tree's roots to its leafy crown.
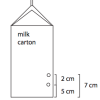
Pressing Pressure
Source Institutions
In this activity, learners compare water pressure at different depths. Learners discover that water pressure increases with depth.

Supercooled Water Drops
Source Institutions
In this activity, learners touch supercooled water drops with an ice crystal and trigger the water drops to freeze instantly.

Moving On Up: Capillary Action II
Source Institutions
Learners explore capillary action in plants (such as plants ability to move water from roots to leaves) in an investigation called Paper Blooms.

Measure the Speed of a Water Leak
Source Institutions
In this activity (page 2 of PDF under GPS: Glaciers Activity), learners will measure the rate at which water streams out of a leaky cup.

What's So Special about Water: Surface Tension
Source Institutions
In this three-part activity, learners play a game and conduct two simple experiments to explore water and surface tension. Learners will have fun discovering how water "sticks" together.

Above Water: Buoyancy & Displacement
Source Institutions
In an investigation called "Shape It!" learners craft tiny boats out of clay, set them afloat on water and then add weight loads to them, in order to explore: how objects stay afloat in water; what th

Using Color to See How Liquids Combine
Source Institutions
Learners add different liquids (water, salt water, alcohol, and detergent solution) to water and observe the different ways the different liquids combine with water.
