Search Results
Showing results 41 to 60 of 140

Iron in the Environment
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners corrode a penny in a cup with vinegar, salt water, and a source of iron (nails, paper clips, or twist ties).
Current Events
Source Institutions
Learners model the ocean currents that carry hot water from the tropics to northern latitudes.
Up and Over
Source Institutions
This is an activity about Newton's First Law of Motion - a body in motion tends to stay in motion, or a body at rest tends to stay at rest unless acted upon by an outside force.

Egg Osmosis: A four day eggsperience!
Source Institutions
Eggs are placed in vinegar for one or two days to dissolve the shells. Then, learners place the eggs in water or corn syrup and observe them over a period of days.

Evaporation
Source Institutions
This three-part activity consists of an activity that groups of learners develop themselves, a given procedure, and an optional demonstration.

Moving Without Wheels
In a class demonstration, learners observe a simple water cycle model to better understand its role in pollutant transport.

Racing M&M Colors
Source Institutions
Learners design their own experiment to determine which M&M color dissolves the fastest in water.

Convection Demonstration
Source Institutions
In this quick activity (located on page 2 of the PDF under GPS: Balloon Fiesta Activity), learners will see the effects of convection and understand what makes hot air balloons rise.

Collect Invertebrates to Determine Water Quality
Source Institutions
This activity (located on page 3 of the PDF under GPS: Alligator Habitat Activity) is a full inquiry investigation into organisms and the health of their ecosystems.

Turbidity
Source Institutions
This is an activity about turbidity, or the amount of sediment suspended in water.

Diving Submarine
Source Institutions
Learners use a commercially available toy to experiment with density. They fill a chamber in the toy submarine with baking powder and release it into a tank of water.

Oh Boy Buoyancy
Source Institutions
In this physics activity, learners will explore the concept of buoyancy, especially as it relates to density.

"Can" You Stand the Pressure
Source Institutions
In this activity about states of matter, learners get to witness firsthand the awesome power of air pressure. They watch as an ordinary soda can is crushed by invisible forces.

Exploring Products: Nano Sand
Source Institutions
In this activity, learners explore how water behaves differently when it comes in contact with "nano sand" and regular sand.

Exploring Moisture on the Outside of a Cold Cup
Source Institutions
In this activity, learners explore the relationship between cooling water vapor and condensation. Learners investigate condensation forming on the outside of a cold cup.

Shark Sense of Smell
Source Institutions
This is an activity about our sense of smell and how it compares to sharks' super noses. Learners will create varying solutions of water and perfume.

Changing the Density of an Object: Changing Shape
Source Institutions
Learners will see that changing the shape of an object, like a clay ball, that is more dense than water, can affect whether the object will sink or float.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.
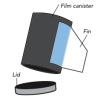
Pop Rockets
Source Institutions
In this activity, learners make film canister rocket ships. A fin pattern is glued onto the outside of the canister, and fuel (water and half an antacid tablet) is mixed inside the canister.

Changing the Density of an Object: Adding Material
Source Institutions
Learners see that a can of regular cola sinks while a can of diet cola floats. As a demonstration, bubble wrap is taped to the can of regular cola to make it float.
