Search Results
Showing results 61 to 80 of 140
Snow Day!
Source Institutions
In this activity (on pages 4-5), learners make fake snow by adding water to the super-absorbant chemical from diapers, sodium polyacrylate.

See It to Believe It: Visual Discrimination
Source Institutions
In this activity (12th on the page), learners investigate their ability to discriminate (see) different colors.

Identifying Erosion
Source Institutions
In this environmental science activity (page 3 of the PDF), leaners will identify and explain the causes of erosion.

Look-alike Liquids
Source Institutions
Learners add drops of four liquids (water, alcohol, salt water, and detergent solution) to different surfaces and observe the liquids' behavior.

M&M's in Different Temperatures
Source Institutions
Learners design their own experiment to investigate whether the temperature of the surrounding water affects the rate at which the colored coating dissolves from an M&M.
Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).

M&M's in Different Sugar Solutions
Source Institutions
In this activity, learners investigate whether having sugar already dissolved in water affects the speed of dissolving and the movement of sugar and color through the water.
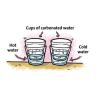
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Solving Dissolving
Source Institutions
The Sacred Cenote at Chichén Itzá is a sink hole, or well, containing groundwater. In this activity, learners create their own cenote using chalk, limestone, acids, and rain water.

Make a Lake
Source Institutions
Where rainwater goes after the rain stops? And why there are rivers and lakes in some parts of the land but not in others?
Bend a Carrot
Source Institutions
In this activity, learners investigate the process of osmosis by adding salt to a sealed bag of raw carrots and comparing it to a control.

Hot Stuff!: Creating and Testing for Carbon Dioxide
In this demonstration, learners observe vinegar and baking soda reacting to form carbon dioxide (CO2) gas.

Solubility Test
Source Institutions
In this activity, learners apply a dissolving test to known crystals to identify the unknown. Since the unknown is chemically the same as one of the known crystals, it should dissolve similarly.
Heat Speeds Up Reactions
Source Institutions
In this activity, learners investigate the effect of heat on a reaction.

Matter on the Move
Source Institutions
Learners observe and conduct experiments demonstrating the different properties of hot and cold materials.
Atoms and Matter (3-6)
Source Institutions
In this activity, learners build models of atoms and molecules, then consider their role in different phases of matter, density, and mixtures and solutions.

Wetlands
Source Institutions
Learners create a model of a wetland to observe how it absorbs and filters water from the environment.

Polishing Pennies
Source Institutions
In this experiment, learners try different liquids to see which ones clean pennies best. Liquids to try include water, lemon juice, cola, vinegar, and dishwashing detergent.

Fill 'er Up!
Source Institutions
Learners discover that their breath contains carbon dioxide, one of the pollutants found in car exhaust.

Eyedropper Hydrometer: Buoy your understanding of density
Source Institutions
Build a hydrometer (measures the density of a liquid) using a pipet or eyedropper.
