Search Results
Showing results 301 to 320 of 619
Foam Peanuts
Source Institutions
Learners compare the properties and solubilities of Styrofoam (TM), ecofoam packing peanuts, and popcorn. First, the solubility of each substance is tested in water.

Defining Dissolving
Source Institutions
In this introductory activity, learners discover that sugar and food coloring dissolve in water but neither dissolves in oil.

Phase Changes
Source Institutions
Learners observe a sealed test tube containing a small amount of solid stearic acid.
What Does Life Need to Live?
Source Institutions
In this astrobiology activity (on page 11 of the PDF), learners consider what organisms need in order to live (water, nutrients, and energy).

Which Powder is It?
Source Institutions
In this chemistry challenge, learners identify an unknown white powder by comparing it with common household powders.

Plant Power
Source Institutions
In this chemistry challenge, learners identify which plants have the enzyme "catalase" that breaks hydrogen peroxide into water and oxygen.
Egg Osmosis
Source Institutions
Visitors observe three beakers. One beaker contains an egg immersed in vinegar. Visitors observe carbon dioxide gas escaping from the shell as the calcium carbonate reacts with the vinegar.

Weighty Questions
Source Institutions
In this activity about humans and space travel (page 1 of PDF), learners compare and contrast the behavior of a water-filled plastic bag, both outside and inside of a container of water.

A Dissolving Challenge
Source Institutions
In this activity, learners add objects and substances to carbonated water to discover that added objects increase the rate at which dissolved gas comes out of solution.
Investigating Convection
Source Institutions
This experiment is designed to illustrate how fluids, including water, have the ability to flow.

Lotus Leaf Effect
Source Institutions
This is a demonstration about how nature inspires nanotechnology. It is easily adapted into a hands-on activity for an individual or groups.

Challenge: Microgravity
Source Institutions
In this activity about the circulatory system and space travel (on page 38 of the PDF), learners use water balloons to simulate the effects of gravity and microgravity on fluid distribution in the bod

Dunk and Flip
Source Institutions
Learners complete two simple experiments to prove the existence of air and air pressure which surround us.

Half Full or Half Empty
Source Institutions
In this activity (12th activity on the page), learners conduct an experiment to demonstrate how muscles are constantly feeding information to the brain about what they are doing.
How is Coastal Temperature Influenced by the Great Lakes and the Ocean?
Source Institutions
In this two-part lesson, learners discover how large bodies of water can serve as a heat source or sink at different times and how proximity to water moderates climate along the coast.

Test Density with a Supersaturated Solution
Source Institutions
Learners create three solutions with different levels of salinity. They compare the density of these solutions by coloring them and layering them in a clear plastic cup and in a soda bottle.
Gummy Growth
Source Institutions
In this activity related to Archimedes' Principle, learners use water displacement to compare the volume of an expanded gummy bear with a gummy bear in its original condition.

Crunch Time
Source Institutions
In this quick and easy activity and/or demonstration, learners use two empty 2-liter bottles and hot tap water to illustrate the effect of heat on pressure.
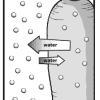
Bend a Carrot
Source Institutions
In this activity, learners investigate the process of osmosis by adding salt to a sealed bag of raw carrots and comparing it to a control.

Shrinking Cups
Source Institutions
This is a quick activity (on page 2 of the PDF under Gecko Feet Activity) about the forces of gravity and surface tension and how their behavior is influenced by size.
