Search Results
Showing results 1 to 20 of 22

Heat Capacity: Can't Take the Heat?
Source Institutions
Why is ocean water sometimes the warmest when the average daily air temperature starts to drop? In this activity, learners explore the differing heat capacities of water and air using real data.

Differing Densities: Fresh and Salt Water
Source Institutions
In this activity, learners visualize the differences in water density and relate this to the potential consequences of increased glacial melting.

Solar Water Heater
Learners work in teams to design and build solar water heating devices that mimic those used in residences to capture energy in the form of solar radiation and convert it to thermal energy.

Cool It!
Source Institutions
Learners make a refrigerator that works without electricity. The pot-in-pot refrigerator works by evaporation: a layer of sand is placed between two terra cotta pots and thoroughly soaked with water.

Causes and Effects of Melting Ice
Source Institutions
In this activity, learners explore the concept of density-driven currents (thermohaline circulation) and how these currents are affected by climate change.

What Causes Wind?
Source Institutions
In this sunny day experiment, learners measure and compare how quickly light and dark colored materials absorb heat.
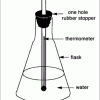
Using Solar Energy
Source Institutions
In this activity, learners discover how solar energy can be used to heat water.
Finding the Right Crater
Source Institutions
This quick demonstration (on page 11 of PDF) allows learners to understand why scientists think water ice could remain frozen in always-dark craters at the poles of the Moon.

Global Climate Change and Sea Level Rise
Source Institutions
In this activity, learners practice the steps involved in a scientific investigation while learning why ice formations on land (not those on water) will cause a rise in sea level upon melting.
Cooling the Mummy's Tomb
Source Institutions
In this activity, learners conduct an experiment to help Pharaoh design a better insulated tomb.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Making An Impact!
Source Institutions
In this activity (on page 14 of PDF), learners use a pan full of flour and some rocks to create a moonscape.
The Dead Zone: A Marine Horror Story
Source Institutions
In this environmental science and data analysis activity, learners work in groups to track a Dead Zone (decreased dissolved oxygen content of a body of water) using water quality data from the Nutrien

Hot Cans and Cold Cans
Source Institutions
Learners apply their knowledge of heat transfer to design two cans - one that will retain heat and one that will cool down quickly.

Feel the Heat
Source Institutions
In this design challenge activity, learners design and build a solar hot water heater. Their goal is to create a heater that yields the highest temperature change.

Do Cities Affect the Weather?
Source Institutions
In this activity, learners explore clouds and how they form.

Sea Level: On The Rise
Source Institutions
Learners will understand the relationship between climate change and sea-level rise.

Sugar Crystal Challenge
Source Institutions
This lesson focuses on surface area and how the shape of sugar crystals may differ as they are grown from sugars of different coarseness.

Pot-in-Pot Refrigeration
Source Institutions
In this activity (on page 2 of PDF), learners create a low-tech refrigerator that requires no electricity to keep food from spoiling.

Keep it Cool
Source Institutions
In this activity, learners explore how engineers have met the challenge of keeping foods, liquids, and other items cool.
