Search Results
Showing results 1 to 20 of 43

Cleaning Water with Dirt
Source Institutions
In this activity on page 7 of the PDF (Water in Our World), learners make their own water treatment systems for cleaning water.

Water Treatment
Source Institutions
Water treatment on a large scale enables the supply of clean drinking water to communities.

Foam Tower
Source Institutions
In this activity (page 1 of the PDF under SciGirls Activity: Water Slides), learners will whip up some suds with a cup of water and a tablespoon of dish soap until the bubbles are stiff enough to star
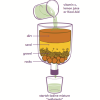
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Causes and Effects of Melting Ice
Source Institutions
In this activity, learners explore the concept of density-driven currents (thermohaline circulation) and how these currents are affected by climate change.

Indicating Electrolysis
Source Institutions
Electrolysis is the breakdown of water into hydrogen and oxygen. This Exploratorium activity allows learners to visualize the process with an acid-based indicator.

Fragile Waters
Source Institutions
In this activity (on pages 18-29) learners explore the impact of the March 24, 1989 oil spill in Alaska caused by the Exxon Valdez tanker.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.
Marshmallow Models
Source Institutions
No glue is needed for learners of any age to become marshmallow architects or engineers.

Mystery Sand
Source Institutions
In this activity, learners play with surprising sand that doesn’t get wet! Learners explore how water behaves differently when it comes in contact with "magic sand" and regular sand.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.

Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.

Human Impact on Estuaries: A Terrible Spill in Grand Bay
Source Institutions
In this activity, learners make a model of a pollution spill that occurred at Bangs Lake in Mississippi and measure water quality parameters in their model.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.

Ocean in a Bottle
Source Institutions
In this activity, learners consider how oil spills behave in the ocean and what impact they have on marine wildlife.

Foam Peanuts
Source Institutions
Learners compare the properties and solubilities of Styrofoam (TM), ecofoam packing peanuts, and popcorn. First, the solubility of each substance is tested in water.
What Does Life Need to Live?
Source Institutions
In this astrobiology activity (on page 11 of the PDF), learners consider what organisms need in order to live (water, nutrients, and energy).

A Dissolving Challenge
Source Institutions
In this activity, learners add objects and substances to carbonated water to discover that added objects increase the rate at which dissolved gas comes out of solution.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

Fill 'er Up!
Source Institutions
Learners discover that their breath contains carbon dioxide, one of the pollutants found in car exhaust.
