Search Results
Showing results 1 to 20 of 28

Cleaning Water with Dirt
Source Institutions
In this activity on page 7 of the PDF (Water in Our World), learners make their own water treatment systems for cleaning water.
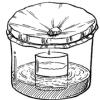
Rain Machine (Solar Still)
Source Institutions
In this activity, learners work in groups to build simple solar stills filled with salt water. After the stills are complete, learners observe what happens when they place the stills in the sun.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.

Exploring Materials: Hydrogel
Source Institutions
In this activity, learners discover how a super-absorbing material can be used to move a straw.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.

Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.

Snow Day!
Source Institutions
In this activity (on pages 4-5), learners make fake snow by adding water to the super-absorbant chemical from diapers, sodium polyacrylate.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Build A Hydrometer
Source Institutions
In this activity, learners will explore how a hydrometer works by building a working model and conducting experiments.
Egg Osmosis
Source Institutions
Visitors observe three beakers. One beaker contains an egg immersed in vinegar. Visitors observe carbon dioxide gas escaping from the shell as the calcium carbonate reacts with the vinegar.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

Chemical Footprint—Family Activity
Source Institutions
In this multi-part activity learners examine non-point water pollution.

Rocket Science
Source Institutions
Learners create a small explosion by collecting hydrogen and oxygen gas together and squeezing them into a flame.

Playtime Paint
Source Institutions
In this activity on page 9 of the PDF, learners make their own paint using chalk as a pigment and glue and water as binders. This activity introduces learners to special mixtures called suspensions.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.

Exploring Materials: Thin Films
Source Institutions
In this activity, learners create a colorful bookmark using a super thin layer of nail polish on water. Learners discover that a thin film creates iridescent, rainbow colors.

Avi's Sensational Salt Dough
Source Institutions
In this activity on page 5 of the PDF, learners mimic the process for making bricks. Learners shape and bake creations from a dough that is made from flour, salt, and water.

Liquid Body Armor
Source Institutions
In this activity, learners explore how nanotechnology is being used to create new types of protective fabrics.
Urine the Know
Source Institutions
In this activity on page 5 of the PDF, learners compare water with artificial urine to see how urinalysis works. Learners use urinalysis test strips to test for glucose and protein in the fake urine.
