Search Results
Showing results 1 to 20 of 71

Comparing the Density of an Object to the Density of Water
Source Institutions
Learners compare the weight of equal volumes of wax, water, and clay. Learners discover that since the wax weighs less than an equal volume of water, it is less dense than water and will float.

Make a Wire Critter That Can Walk on Water
Source Institutions
In this activity, learners make water-walking critters using thin wire, and then test how many paper clips these critters can carry without sinking.
Pressing Pressure
Source Institutions
In this activity, learners compare water pressure at different depths. Learners discover that water pressure increases with depth.

Differing Densities: Fresh and Salt Water
Source Institutions
In this activity, learners visualize the differences in water density and relate this to the potential consequences of increased glacial melting.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.

Above Water: Buoyancy & Displacement
Source Institutions
In an investigation called "Shape It!" learners craft tiny boats out of clay, set them afloat on water and then add weight loads to them, in order to explore: how objects stay afloat in water; what th

Pepper Scatter
Source Institutions
In this quick activity, learners break the tension that happens when water develops a "skin." Learners use water, pepper and some soap to discover the wonders of surface tension—the force that attract
Floating Paperclip and Other Surface Tension Experiments
Source Institutions
In this activity, learners experiment with surface tension using everyday household items such as strawberry baskets, paperclips, liquid dish soap, and pepper.

How Much Water is in that Cloud?
Source Institutions
In this activity, learners working in pairs saturate a cotton ball using water drops from an eyedropper to demonstrate the high water capacity of clouds.
Dollar Bill Grab
Source Institutions
In this demonstration, learners observe as two cola bottles and a dollar bill are arranged in a specific order: one bottle, upside down and filled with water, is placed on top of another bottle, with

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!

Diet Light
Source Institutions
In this quick activity, learners observe how the added sugar in a can of soda affects its density and thus, its ability to float in water.
Under Pressure
Source Institutions
In this experiment, learners examine how pressure affects water flow. In small groups, learners work with water and a soda bottle, and then relate their findings to pressure in the deep ocean.

Free-Fall Bottles & Tubes
Source Institutions
In this physics activity, learners conduct two experiments to explore free-falling.

Dye Detective
Source Institutions
Learners analyze mixtures of dyes using filter paper chromatography. They place spots of the different dyes at the bottom of a piece of filter paper, and hang the paper to touch the surface of water.
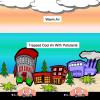
Turning the Air Upside Down: Convection Current Model
Learners see convection currents in action in this highly visual demonstration. Sealed bags of colored hot or cold water are immersed in tanks of water.
Aluminum Boats
Source Institutions
Test the buoyancy of an aluminum foil boat and an aluminum foil ball. Why does the same material in different shapes sink or float?
Submersibles and Marshmallows
Source Institutions
In this activity, learners discover the difficulty of ocean exploration by human beings as they investigate water pressure.

Design a Submarine
Source Institutions
Learners act as engineers and design mini submarines that move in the water like real submarines.

What is in the Water?
Source Institutions
In this activity, learners use open inquiry to learn about the process of science as well as gain experience regarding the Law of Conservation of Mass, dissolution, and density.
