Search Results
Showing results 1 to 20 of 25

Mystery Powders
Source Institutions
Learners are given mysterious white powders and have to determine their identity with chemical tests.

3-2-1 POP!
Source Institutions
In this physics activity, learners build their own rockets out of film canisters and construction paper.

The Gas You Pass
Source Institutions
Although we may not admit it, all humans fart or pass some gas. In this activity, learners make their own model to mimic food passing through intestines and discover what releases gas.

Earth Atmosphere Composition
Source Institutions
In this activity, learners use rice grains to model the composition of the atmosphere of the Earth today and in 1880. Learners assemble the model while measuring percentages.

Inflate-a-mole
Source Institutions
In this activity, learners conduct an experiment to find the volume of one mole of gas. Learners capture sublimated gas from dry ice in a ziploc bag and use water displacement to measure its volume.

Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

Balloon in a Flask
Source Institutions
Learners observe a flask with a balloon attached over the mouth and inverted inside the flask.

Exploring the Universe: Nebula Spin Art
Source Institutions
In this activity, participants will learn about how gigantic clouds of gas and dust in space, called nebulas, are formed. They'll create their own colorful model nebula using paint and a spinner.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Build a Rocket - and a Launch Pad!
Source Institutions
In this activity, learners construct a rocket powered by the pressure generated from an effervescing antacid tablet reacting with water, and build a launch pad for their rocket.

Exploring the Universe: Star Formation
Source Institutions
In this activity, participants will learn how stars form from the dust and gas that exists in space clumping together.

Light Soda
Source Institutions
In this activity, learners sublimate dry ice and then taste the carbon dioxide gas.

It's a Gas!
Source Institutions
In this activity, learners explore two properties of gases: gases take up space and exert pressure. Learners assemble two flasks and a beaker, connecting them with stoppers and tubing.
Stability of Egg White Foams
Source Institutions
In this chemistry meets cooking activity, learners compare the stability of egg white foams with various additives.

Conversation Piece
Source Institutions
Focus sound through a balloon! In this Exploratorium activity, you'll use dry ice to create a balloon that's a sound lens.

Got Gas?
Source Institutions
Create gas with a glass of water, some wire, conductors and a battery! You will be separating water (H2O) into oxygen and hydrogen.

Pop Rockets
Source Institutions
Learners place water and part of an antacid tablet in a film canister. The reaction creates a gas reaction that launches the film canister like a rocket.
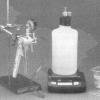
As Light as Air
Source Institutions
Learners measure a bottle full of air, and then use a vacuum pump to remove the air. When they re-weigh the bottle, learners find the mass is about 0.8g less.

Pot-in-Pot Refrigeration
Source Institutions
In this activity (on page 2 of PDF), learners create a low-tech refrigerator that requires no electricity to keep food from spoiling.

A Mole of Gas
Source Institutions
In this two-part activity, learners use everyday materials to visualize one mole of gas or 22.4 liters of gas. The first activity involves sublimating dry ice in large garbage bag.
