Search Results
Showing results 21 to 40 of 45

Chemistry in the Kitchen
Source Institutions
In this kitchen chemistry activity, learners explore the chemistry of crystals by making sugar crystals, consider a common chemical reaction type responsible for the rising of muffins and cake in the

Mystery Shapes
Source Institutions
In this activity, learners describe an object they can’t see. After someone picks outs a few mystery objects and places them in a pillowcase, learners will investigate using only their hands.

The Ups and Downs of Thermometers
Source Institutions
In this activity, learners examine the parts of a thermometer. After placing a thermometer in hot and cold water, learners look at molecular model animations of the liquid in a thermometer.

Battling for Oxygen
Working in groups, learners model the continuous destruction and creation of ozone (O3) molecules, which occur in the ozone layer.

Air, It's Really There
Source Institutions
This lesson focuses on molecular motion in gases. Learners compare the mass of a basketball when it is deflated and after it has been inflated.

Changing Colors
Source Institutions
Learners experiment with a commercially available liquid-crystal coaster. They warm the material with their hands for varying lengths of time and observe the changing colors that result.

Stained Glass Glue
Source Institutions
In this activity on page 6 of the PDF, learners use glue instead of glass to create artwork that can be hung in a window.

Magic Colored Milk
Source Institutions
In this chemistry activity (page 5 of the PDF), learners will use milk and a few other basic ingredients to create a chemical change to make a color wheel.

Air Pressure
Source Institutions
In this experiment, learners use a blow dryer and water bottle to observe and record changes in air pressure caused by changes in temperature.

Make Your Own Soda Pop
Source Institutions
In this chemistry activity (page 8 of the PDF), learners will identify the instances of physical change, chemical change, and solutions while making homemade soda pop.

Exploring Forces: Gravity
Source Institutions
In this nanoscience activity, learners discover that it's easy to pour water out of a regular-sized cup, but not out of a miniature cup.

The Carbon Cycle and its Role in Climate Change: Activity 1
Source Institutions
In this activity (on page 1), learners role play as atoms to explore how atoms can be rearranged to make different materials.

Shake and Make: Charge Recognition
Source Institutions
In this activity (page 10), learners explore how molecules self-assemble according to forces of attraction and repulsion.

Mighty Molecules
Source Institutions
In this activity, learners use marshmallows and gum drops to construct seven models of molecules. Learners classify (solid, liquid or gas) and draw diagrams of the molecules.

Disease Detectives
Source Institutions
In this activity, learners examine antibodies and antibody recognition using a model.

Gas Model
Source Institutions
This highly visual model demonstrates the atomic theory of matter which states that a gas is made up of tiny particles of atoms that are in constant motion, smashing into each other.
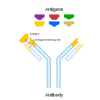
3D-ection: Molecular Shape Recognition
Source Institutions
In this activity (page 12), learners explore how molecules self-assemble and how molecules must fit together, like a lock and key, in order to identify each other and initiate a new function as a comb

A Slime By Any Other Name
Source Institutions
This fun video explains how to make a batch of oobleck (or slime) and why this special substance is known as a "non-Newtonian" fluid. Watch as Mr.

Inverted Bottles
Source Institutions
In this activity, learners investigate convection by using food coloring and water of different temperatures.
Traveling Nanoparticles Model
Source Institutions
This is an activity (located on page 3 of the PDF under Nanosilver Activity) about diffusion of small molecules across cell membranes.
