Search Results
Showing results 1 to 20 of 45

Metal Reactions
Source Institutions
This is written as a static display, but can easily be adapted to a hands-on experiment for learners to conduct.

Latent Prints
Source Institutions
In this activity, learners examine fingerprints. Learners leave a hidden print on a surface and then make their own print detecting powder from graphite (found in pencils).

Mystery Powders
Source Institutions
Learners are given mysterious white powders and have to determine their identity with chemical tests.

Divers
Source Institutions
Learners experiment with a 2-liter plastic bottle containing water and four “divers." The divers consist of open, transparent containers with the opening points downward.

Odors Aloft
Source Institutions
Learners smell balloons filled with different scents to guess what's inside. From this, they infer the presence and motion of scented molecules.
Quipus
Source Institutions
Learners create an Incan counting device called a quipu (pronounced kee-poo).

Mystery Writing: Write and develop a secret message
Source Institutions
Learners write an invisible message using lemon juice on a piece of paper. They then develop the message by soaking the paper in a dilute iodine solution.

Color Me Blue
Source Institutions
In this activity, learners add dilute bleach solution to water that has been dyed with yellow, blue, and green food color.

Dusting For Fingerprints
Source Institutions
In this activity, learners become detectives and use chemistry to investigate fingerprints.
Penny for Your Thoughts
Source Institutions
In this activity, learners will explore how metals react with each other. They will see these metals change before their eyes as they coat a paperclip with the copper taken from a penny.

Matter of Degree
Source Institutions
In two separate bags, learners mix water with Epsom salts and detergent.

Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

Starch Breakdown
Source Institutions
Learners use Benedict’s solution and heat to test for the presence of simple sugars in glucose, sucrose, starch, and starch combined with amylase.
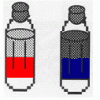
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.
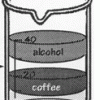
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.

Inner Space
Source Institutions
In this activity, learners discover that there is space between molecules even in a cup "full" of water. They first fill a cup with marbles, and then add sand to fill the gaps between the marbles.

The Best Dam Simulation Ever
Source Institutions
This online simulation game explores the different consequences of water levels on the Columbia River in the Pacific Northwest.

Invisible Ink
Source Institutions
In this hands-on activity (on page 2 of the PDF), learners experiment with lemon juice and paper to create a message that can only be revealed using chemistry.

Program a Friend
Source Institutions
In this activity (on page 2), one person "programs" the other like a robot to move through a space, trying to get them to avoid obstacles and reach a goal.

Acting Out
Source Institutions
This activity (on pages 21-32 of PDF) has learners act out several classic brain teasers.
