Search Results
Showing results 1 to 18 of 18

Collect Oxygen Over Water
Source Institutions
In this activity, learners use a pneumatic trough (see related activity) to generate and collect pure oxygen.
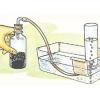
Pneumatic Trough
Source Institutions
In this activity, learners build a "pneumatic trough," a laboratory apparatus used for collecting pure gas samples over water.

The Gas You Pass
Source Institutions
Although we may not admit it, all humans fart or pass some gas. In this activity, learners make their own model to mimic food passing through intestines and discover what releases gas.

Earth Atmosphere Composition
Source Institutions
In this activity, learners use rice grains to model the composition of the atmosphere of the Earth today and in 1880. Learners assemble the model while measuring percentages.

Inflate-a-mole
Source Institutions
In this activity, learners conduct an experiment to find the volume of one mole of gas. Learners capture sublimated gas from dry ice in a ziploc bag and use water displacement to measure its volume.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Build a Rocket - and a Launch Pad!
Source Institutions
In this activity, learners construct a rocket powered by the pressure generated from an effervescing antacid tablet reacting with water, and build a launch pad for their rocket.

Exploring the Universe: Star Formation
Source Institutions
In this activity, participants will learn how stars form from the dust and gas that exists in space clumping together.

Light Soda
Source Institutions
In this activity, learners sublimate dry ice and then taste the carbon dioxide gas.

Got Gas?
Source Institutions
Create gas with a glass of water, some wire, conductors and a battery! You will be separating water (H2O) into oxygen and hydrogen.

Pop Rockets
Source Institutions
Learners place water and part of an antacid tablet in a film canister. The reaction creates a gas reaction that launches the film canister like a rocket.

Having a Gas with Water
Source Institutions
In this activity, learners construct a simple electrolysis device. With this device, learners can decompose water into its elemental components: hydrogen and oxygen gas.

Sizing Up Temperature
Source Institutions
In this activity, learners explore Charles' Law in a syringe.

A Mole of Gas
Source Institutions
In this two-part activity, learners use everyday materials to visualize one mole of gas or 22.4 liters of gas. The first activity involves sublimating dry ice in large garbage bag.

Backyard Biodiesel
Source Institutions
In this activity, learners make a small batch of biodiesel that will work in any diesel engine. Learners use an old juice bottle as a "reactor" vessel to chemically process vegetable oil into fuel.

Having a Gas with Cola
Source Institutions
In this activity, learners measure the amount of carbon dioxide in a carbonated drink.

Yeast Balloons: Can biochemistry blow up a balloon?
Source Institutions
Using yeast, sugar, and water, learners create a chemical reaction which produces carbon dioxide (CO2) gas inside a 2-liter bottle. They use this gas to inflate a balloon.

Hot Air Balloon
Source Institutions
In this activity, learners build a hot air balloon using just a few sheets of tissue paper and a hair dryer.
