Search Results
Showing results 1 to 20 of 37
Trading Places: Redox Reactions
Source Institutions
Visitors add drops of copper sulfate solution onto a steel nail. They observe the nail change color from silver to brown as the copper plates onto the nail.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

Cartesian Diver
Source Institutions
In this demonstration, learners observe the effects of density and pressure. A "diver" constructed out of a piece of straw and Blu-Tack will bob inside a bottle filled with water.

Natural Buffers
Source Institutions
Learners use a universal indicator to test the amount of sodium hydroxide needed to change the pH of plain water compared with the amount needed to change the pH of gelatin.

Open Heart: Disease and Diagnosis
Source Institutions
In this online activity about heart disease, learners will pick one of three patients (based on actual patient histories) and help a cardiologist diagnose and prescribe treatments for him or her.
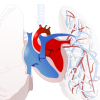
Construct a Heart Circulation Model
Source Institutions
In this online activity about anatomy, learners will correctly order the path blood takes through the heart in order to absorb oxygen to deliver to the rest of the body.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.
Are you a Supertaster?
Source Institutions
In this activity, learners examine their tongue and taste buds.

Experiencing Parallax With Your Thumb
Source Institutions
In this activity, learners investigate parallax, a method used to measure distances to stars and planets in the solar system.
Concentrate: Concentrations and Reaction Rates
Source Institutions
Visitors incrementally increase the amount of iodate in three different test tubes containing the same amount of a starch solution.

Soil Density
Source Institutions
In this activity, learners will test soil content using their sample, some water and a container that seals.

Natural Indicators
Source Institutions
Learners combine different plant solutions -- made from fruits, vegetables, and flowers -- with equal amounts of vinegar (acid), water (neutral), and ammonia (base).

Assemble the Human Heart
Source Institutions
In this online activity about anatomy, learners will drag and drop pieces of the heart into their proper positions and explore what function each part of the heart has.

Proprioception: Wiggle where you're at
Source Institutions
We're told from a young age that we have 5 senses, but we have many more. One of which is our awareness of our own body part's orientation and position.

Michelle O (formerly Vanna)
Source Institutions
We don't normally view people upside down and so our brains aren't accustomed to it.

