Search Results
Showing results 21 to 40 of 65

What's Your Blood Type?
Source Institutions
In this activity, learners perform a simulated blood test procedure.
Shrinkers
Source Institutions
Visitors use heat to shrink samples of polystyrene. They compare samples from containers that were shaped in different ways during manufacturing.
Concentrate: Concentrations and Reaction Rates
Source Institutions
Visitors incrementally increase the amount of iodate in three different test tubes containing the same amount of a starch solution.

Dye Detective
Source Institutions
Learners analyze mixtures of dyes using filter paper chromatography. They place spots of the different dyes at the bottom of a piece of filter paper, and hang the paper to touch the surface of water.

Rocket Science
Source Institutions
Learners create a small explosion by collecting hydrogen and oxygen gas together and squeezing them into a flame.

Balloon in a Flask
Source Institutions
Learners observe a flask with a balloon attached over the mouth and inverted inside the flask.

Starch Breakdown
Source Institutions
Learners use Benedict’s solution and heat to test for the presence of simple sugars in glucose, sucrose, starch, and starch combined with amylase.

Natural Indicators
Source Institutions
Learners combine different plant solutions -- made from fruits, vegetables, and flowers -- with equal amounts of vinegar (acid), water (neutral), and ammonia (base).

Magic Inks
Source Institutions
Learners write their initials by applying different clear "magic ink" solutions to separate pieces of paper and then "develop" the inks with other clear solutions.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.

Shrinkers
Source Institutions
In this hands-on activity, learners use heat to shrink samples of polystyrene plastic (#6 recycle code). Learners compare the size and shape of the plastic pieces before and after shrinking.

Concentrate!
Source Institutions
In this investigation of reaction kinetics, learners alter the amount of iodate solution mixed with the same amount of starch solution.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).

Flubber
Source Institutions
Learners experiment with a piece of Silly Putty® by stretching, bouncing, and snapping it. They then create flubber, a similar substance, by mixing diluted glue and a solution of sodium borate.
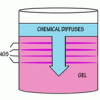
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

Crocodiles
Source Institutions
Learners observe and compare the sizes of three toy “growing” crocodiles made from water-absorbent polymers. One is it its original state, dry, hard, and about 10cm long.

Electroplating
Source Institutions
In this activity, learners electrically plate zinc onto brass objects.

Thar She Glows!
Source Institutions
Learners observe glow-in-the-dark objects in a homemade light-proof box. Objects can include glow sticks, glow-in-the-dark toys, and toys with fluorescent paint.

Acid Rain
Source Institutions
In this chemistry demonstration, acid rain is simulated in a petri dish.

