Search Results
Showing results 1 to 20 of 43
Trading Places: Redox Reactions
Source Institutions
Visitors add drops of copper sulfate solution onto a steel nail. They observe the nail change color from silver to brown as the copper plates onto the nail.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).

Hot and Cold
Source Institutions
In this chemistry challenge, learners discover that many chemical reactions involve heat loss or gain.
The Sharp Eyes of a Naturalist
Source Institutions
In this creative activity, learners will practice looking carefully to observe details and to become familiar with dioramas as a method for displaying naturalistic scenes.

Disappearing Colors
Source Institutions
In this challenge, learners figure out how to make a juice stain disappear.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.

Scavenger Hunt
Source Institutions
An outdoor scavenger hunt helps learners consider the theme of "What Is Life?" Learners explore what living organisms are, including how organisms meet basic needs of food, shelter and water to surviv

DNA Nanotechnology
Source Institutions
In this activity, learners explore deoxyribonucleic acid (DNA), a nanoscale structure that occurs in nature.

Egg-Citing Physics
Source Institutions
In this demonstration about momentum, use physics to distinguish between a hard-boiled egg and a raw egg without cracking them open.

Forms of Carbon
Source Institutions
In this activity, educators can demonstrate how the nanoscale arrangement of atoms dramatically impacts a material’s macroscale behavior.

Sound Bingo
Source Institutions
This game is like regular bingo, except the clues are sounds.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.

Got Seaweed?
Source Institutions
In this activity, learners examine the properties of different seaweeds, investigate what happens when powdered seaweed (alginate) is added to water, and learn about food products made with seaweed.
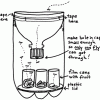
Build a Fruit Fly Trap
Source Institutions
In this construction activity, students use a 2-liter bottle to build a fly trap.
Shrinkers
Source Institutions
Visitors use heat to shrink samples of polystyrene. They compare samples from containers that were shaped in different ways during manufacturing.
Concentrate: Concentrations and Reaction Rates
Source Institutions
Visitors incrementally increase the amount of iodate in three different test tubes containing the same amount of a starch solution.

Animals in a Grassland
Source Institutions
In this outdoor, warm weather activity, learners use sweepnets to search a grassy area such as a large lawn or field, collecting small animals to find as many different kinds of animals as possible.
