Search Results
Showing results 1 to 20 of 30
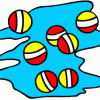
Density: Make a golf ball float!
Source Institutions
In this activity (on page 2 of the PDF), the learner places a golf ball between salt water and colored fresh water. The golf ball is not as dense as the saltwater.
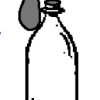
Gas Production: Blow up a balloon!
Source Institutions
In this classic reaction, learners baking soda and vinegar in a soda bottle to produce carbon dioxide (CO2) gas. This gas inflates a balloon.

Kool Colors
Source Institutions
Learners investigate how temperature affects the rate of chemical reactions by observing how steel wool reacts with various types of Kool-Aid solutions at different temperatures.
Odors Aloft
Source Institutions
Learners smell balloons filled with different scents to guess what's inside. From this, they infer the presence and motion of scented molecules.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.

Layered Liquids: Chemistry You Can Drink
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners make a layered drink with liquids of different densities.
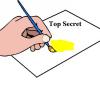
Mystery Writing: Write and develop a secret message
Source Institutions
Learners write an invisible message using lemon juice on a piece of paper. They then develop the message by soaking the paper in a dilute iodine solution.
Of Cabbages and Kings
Source Institutions
This lesson gives full instructions for making cabbage juice indicator, a procedure sheet for learners to record observations as they use the indicator to test materials, and extension activities to d
Matter of Degree
Source Institutions
In two separate bags, learners mix water with Epsom salts and detergent.

Crystals: Grow Your Own Garden
Source Institutions
In this simple activity (on page 2 of the PDF), learners make a crystal garden using salt, water, and a brick.

Changing Colors
Source Institutions
Learners experiment with a commercially available liquid-crystal coaster. They warm the material with their hands for varying lengths of time and observe the changing colors that result.

Shrinkers
Source Institutions
In this hands-on activity, learners use heat to shrink samples of polystyrene plastic (#6 recycle code). Learners compare the size and shape of the plastic pieces before and after shrinking.

Flubber
Source Institutions
Learners experiment with a piece of Silly Putty® by stretching, bouncing, and snapping it. They then create flubber, a similar substance, by mixing diluted glue and a solution of sodium borate.

Recycling Paper
Source Institutions
In this crafty chemistry activity (on page 2 of the PDF), learners make their own paper from used paper they may have otherwise thrown away.
Water Ways
Source Institutions
In this activity (on page 2 of the PDF), learners explore surface tension by adding pennies to cups which are "full" of plain water or soapy water.

Iron in the Environment
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners corrode a penny in a cup with vinegar, salt water, and a source of iron (nails, paper clips, or twist ties).
Inner Space
Source Institutions
In this activity, learners discover that there is space between molecules even in a cup "full" of water. They first fill a cup with marbles, and then add sand to fill the gaps between the marbles.
Plastic Milk: You can make plastic from milk
Source Institutions
In this activity (on page 2 of the PDF), learners make a plastic protein polymer from milk. Adding vinegar to milk causes the protein casein to solidify or curdle.
Invisible Ink
Source Institutions
In this hands-on activity (on page 2 of the PDF), learners experiment with lemon juice and paper to create a message that can only be revealed using chemistry.

Big Things Come in Little Packages
Source Institutions
As a group, learners investigate three packages which are all the same size and shape, but have different contents. One is filled with foam, one is filled with wood, and one is filled with metal.
