Search Results
Showing results 1 to 20 of 21

Divers
Source Institutions
Learners experiment with a 2-liter plastic bottle containing water and four “divers." The divers consist of open, transparent containers with the opening points downward.

Odors Aloft
Source Institutions
Learners smell balloons filled with different scents to guess what's inside. From this, they infer the presence and motion of scented molecules.
Quipus
Source Institutions
Learners create an Incan counting device called a quipu (pronounced kee-poo).

Mystery Writing: Write and develop a secret message
Source Institutions
Learners write an invisible message using lemon juice on a piece of paper. They then develop the message by soaking the paper in a dilute iodine solution.

Matter of Degree
Source Institutions
In two separate bags, learners mix water with Epsom salts and detergent.
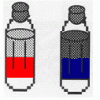
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.

Inner Space
Source Institutions
In this activity, learners discover that there is space between molecules even in a cup "full" of water. They first fill a cup with marbles, and then add sand to fill the gaps between the marbles.

Invisible Ink
Source Institutions
In this hands-on activity (on page 2 of the PDF), learners experiment with lemon juice and paper to create a message that can only be revealed using chemistry.

Program a Friend
Source Institutions
In this activity (on page 2), one person "programs" the other like a robot to move through a space, trying to get them to avoid obstacles and reach a goal.

Big Things Come in Little Packages
Source Institutions
As a group, learners investigate three packages which are all the same size and shape, but have different contents. One is filled with foam, one is filled with wood, and one is filled with metal.

Cabbage Juice Indicator: Test the pH of household products
Source Institutions
Learners make their own acid-base indicator from red cabbage. They use this indicator to test substances around the house.

Shrinkers: Cook up some plastic!
Source Institutions
In this activity (on page 2 of the PDF), learners (with adult help and supervision) investigate how heat affects polystyrene plastic.
Choose Your Ooze
Source Institutions
During this activity, learners will make different versions of "ooze" using varied proportions of detergent and glue.

Shake It Up!
Source Institutions
Learners observe a sealed container holding a clear colorless liquid. They shake the container and the fluid turns blue. When allowed to sit for a few moments, the fluid turns colorless again.

Make a Totem Pole
Source Institutions
In this activity (on page 2 of PDF), learners make their own totem poles out of recycled materials.

Magical Möbius
Source Institutions
In this tabletop activity (on pages 32-40), learners make Möbius strips -- 3D surfaces with only one side.

Paper Whites
Source Institutions
Learners observe different paper samples under ordinary room light and under a black light to learn some of the chemical differences between different types of paper.

Flubber: Make a polymer!
Source Institutions
This activity (on page 2 of the PDF) features a recipe to create the stretchy polymer Flubber from Borax detergent, white glue, and water.

Brick Drop Challenge
Source Institutions
In this design challenge, leaners attempt to build a strong structure out of LEGO® bricks that can withstand a 4-foot drop.

Yeast Balloons: Can biochemistry blow up a balloon?
Source Institutions
Using yeast, sugar, and water, learners create a chemical reaction which produces carbon dioxide (CO2) gas inside a 2-liter bottle. They use this gas to inflate a balloon.
