Search Results
Showing results 1 to 20 of 30

Density: Make a golf ball float!
Source Institutions
In this activity (on page 2 of the PDF), the learner places a golf ball between salt water and colored fresh water. The golf ball is not as dense as the saltwater.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.

Layered Liquids: Chemistry You Can Drink
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners make a layered drink with liquids of different densities.
Penny for Your Thoughts
Source Institutions
In this activity, learners will explore how metals react with each other. They will see these metals change before their eyes as they coat a paperclip with the copper taken from a penny.

Starch Slime
Source Institutions
Learners mix liquid water with solid cornstarch. They investigate the slime produced, which has properties of both a solid and a liquid.

Crystals: Grow Your Own Garden
Source Institutions
In this simple activity (on page 2 of the PDF), learners make a crystal garden using salt, water, and a brick.
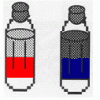
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.

Crocodiles
Source Institutions
Learners observe and compare the sizes of three toy “growing” crocodiles made from water-absorbent polymers. One is it its original state, dry, hard, and about 10cm long.

Three Little Pigs
Source Institutions
In this activity, leaners explore building techniques by recreating the story of The Three Little Pigs.

Water Ways
Source Institutions
In this activity (on page 2 of the PDF), learners explore surface tension by adding pennies to cups which are "full" of plain water or soapy water.

Pearlescent Pigments
Source Institutions
This is written as a display, but can easily be adapted to a hands-on activity. Learners observe and shake containers of shiny liquids.

Iron in the Environment
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners corrode a penny in a cup with vinegar, salt water, and a source of iron (nails, paper clips, or twist ties).

Invisible Ink
Source Institutions
In this hands-on activity (on page 2 of the PDF), learners experiment with lemon juice and paper to create a message that can only be revealed using chemistry.

Acting Out
Source Institutions
This activity (on pages 21-32 of PDF) has learners act out several classic brain teasers.

Shrinkers: Cook up some plastic!
Source Institutions
In this activity (on page 2 of the PDF), learners (with adult help and supervision) investigate how heat affects polystyrene plastic.
Choose Your Ooze
Source Institutions
During this activity, learners will make different versions of "ooze" using varied proportions of detergent and glue.

Miscibility
Source Institutions
Learners observe a bottle containing water and oil. They are invited to pick up the bottle and mix the contents together.

Slide Rules
Source Institutions
Learners make their own simple slide rules out of paper and learn how they work.

Dye Detective
Source Institutions
Learners use filter paper and water to analyze six different markers. They mark the paper with ink, and dip the paper in water. The water travels up the paper and dissolved ink travels with it.

Pot-in-Pot Refrigeration
Source Institutions
In this activity (on page 2 of PDF), learners create a low-tech refrigerator that requires no electricity to keep food from spoiling.
