Search Results
Showing results 121 to 140 of 372

Milk Magic
Source Institutions
In this activity, learners experiment with how dish soap and fat interact by making a colorful swirl.

Marshmallow Puff Tube
Source Institutions
In this demonstration/activity, learners observe as a regular size marshmallow is blown through a tube made from a manila file folder.

Onion DNA Extraction
Source Institutions
This laboratory exercise is designed to show learners how DNA can easily be extracted from onion cells using simple materials.
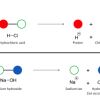
Chemical Identification
Source Institutions
In this activity, learners discover how a cabbage juice indicator helps identify acids and bases, and how iodine indicates the presence of starch.

Milk Makes Me Sick: Exploration of Lactose Intolerance
Source Institutions
Why does milk make some people sick? In this activity learners explore this question and explore the chemistry of milk, and our bodies!

Cheese: Behold the Power of Chemistry
Source Institutions
In this activity on page 7 of the PDF (Get Cooking With Chemistry), learners conduct an experiment to get an idea of how cheese is made.
Why is the Sky Blue?
Source Institutions
In this activity, learners create a "mini sky" in a glass of water in a dark room.

Powder Particulars
Source Institutions
In this introductory activity and demonstration, learners are introduced to the concept that different substances react chemically in characteristic ways.

Cake by Conduction
Source Institutions
In this demonstration, cook a cake using the heat produced when the cake batter conducts an electric current.

Does Your Chewing Gum Lose Its Flavor?
Source Institutions
Each learner chews a piece of gum until it loses its flavor, and then leaves the gum to dry for several days.

Molecular Menagerie
Source Institutions
In this activity, learners use molecular model kits to construct familiar molecules like lactose, caffeine, and Aspirin.

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!

Spaghetti Strength
Source Institutions
In this activity on page 7 of the PDF, learners explore how engineers characterize building materials.

Take an Egg for a Spin
Source Institutions
This is an activity about friction as well as kinetic and potential energy.

Chromatography
Source Institutions
In this chemistry activity, learners will separate a mixture of FD&C dyes (colors certified and allowed by the US for the Food, Pharmaceutical, Cosmetics & Personal Care industry) to practice

Be A Pasta Food Scientist
Source Institutions
In this activity, learners of all ages can become food scientists by experimenting with flour and water to make basic pasta.

Sugar Crystal Challenge
Source Institutions
This lesson focuses on surface area and how the shape of sugar crystals may differ as they are grown from sugars of different coarseness.
Marshmallow Models
Source Institutions
No glue is needed for learners of any age to become marshmallow architects or engineers.

Candy Chromatography
Source Institutions
Learners analyze candy-coated sweets using chromatography. Learners use this method to separate the various dyes used to make colored candy.

Cook with a Solar Oven
Source Institutions
In this activity, learners make their own solar oven to bake s'mores and learn about how solar energy is absorbed on Earth.
