Search Results
Showing results 21 to 40 of 55
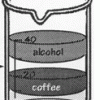
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.

Build A Battery
Source Institutions
The Let's Do Chemistry "Build a Battery" activity lets participants learn how batteries work and how materials behave, change, and interact by building their own simple battery out of metal and felt w
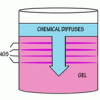
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

Ice Cream Freeze
Source Institutions
In this fun and delicious chemistry activity (page 1 of the PDF), learners will explore the difference between physical and chemical change by making homemade ice cream.

Crocodiles
Source Institutions
Learners observe and compare the sizes of three toy “growing” crocodiles made from water-absorbent polymers. One is it its original state, dry, hard, and about 10cm long.

Resistance is Useful
Source Institutions
Learners write or draw with white crayon on white paper. They look and feel to detect their marks on the paper. Then, learners paint over their paper with watercolor paint.

Pearlescent Pigments
Source Institutions
This is written as a display, but can easily be adapted to a hands-on activity. Learners observe and shake containers of shiny liquids.

Swirling Milk
Source Institutions
In this chemistry activity, learners prepare two petri dishes, one filled with water and one filled with milk.

Sidewalk Chalk
Source Institutions
In this chemistry activity, learners witness an exothermic reaction, while making their very own, completely usable sidewalk chalk. This is also an excellent activity for exploring color mixing.

Rock Candy
Source Institutions
In this yummy chemistry activity which requires adult supervision, learners use sugar and water to explore how crystals form.

Nature of Dye
Source Institutions
"Nature of Dye" allows participants to create their own dyes and art while exploring how chemicals interact and how these interactions can have real-world applications.

Tempest in a Teacup
Source Institutions
In this hands-on activity, learners determine the types of chemical reactions achieved when combining different household products.

To Dye For
Source Institutions
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each.

Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.
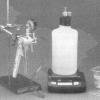
As Light as Air
Source Institutions
Learners measure a bottle full of air, and then use a vacuum pump to remove the air. When they re-weigh the bottle, learners find the mass is about 0.8g less.

Chemistry Makes Scents
Source Institutions
In "Chemistry Makes Scents," participants use their noses to distinguish between chemicals with very similar structures.

Miscibility
Source Institutions
Learners observe a bottle containing water and oil. They are invited to pick up the bottle and mix the contents together.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Chemistry Is Colorful
Source Institutions
In "Chemistry is Colorful" learners explore different materials through paper chromatography.

