Search Results
Showing results 21 to 40 of 41
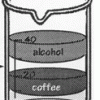
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.
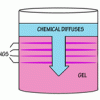
Liesegang Rings
Source Institutions
This display shows slow chemical reactions in colorful crystal formations known as Liesegang Rings. These reactions are similar to those forming the rings in agates.

Crocodiles
Source Institutions
Learners observe and compare the sizes of three toy “growing” crocodiles made from water-absorbent polymers. One is it its original state, dry, hard, and about 10cm long.

Resistance is Useful
Source Institutions
Learners write or draw with white crayon on white paper. They look and feel to detect their marks on the paper. Then, learners paint over their paper with watercolor paint.

Pearlescent Pigments
Source Institutions
This is written as a display, but can easily be adapted to a hands-on activity. Learners observe and shake containers of shiny liquids.

Swirling Milk
Source Institutions
In this chemistry activity, learners prepare two petri dishes, one filled with water and one filled with milk.

To Dye For
Source Institutions
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each.
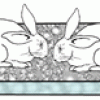
Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.
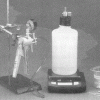
As Light as Air
Source Institutions
Learners measure a bottle full of air, and then use a vacuum pump to remove the air. When they re-weigh the bottle, learners find the mass is about 0.8g less.

Miscibility
Source Institutions
Learners observe a bottle containing water and oil. They are invited to pick up the bottle and mix the contents together.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

See the Light
Source Institutions
Learners mix a solution of luminol with hydrogen peroxide to produce a reaction that gives off blue light.

Bounce vs. Thud Balls
Source Institutions
Learners compare the properties of two balls that appear identical. One ball bounces, while the other ball "thuds." The “bounce” ball is made of the polymer polybutadiene (-C4H4-).

Shake It Up!
Source Institutions
Learners observe a sealed container holding a clear colorless liquid. They shake the container and the fluid turns blue. When allowed to sit for a few moments, the fluid turns colorless again.

Lava Lamps
Source Institutions
Learners observe working lava lamps to understand how they work (included in PDF link).

Electrolysis
Source Institutions
Learners observe two joined glass tubes containing a conductive salt solution. Electrodes are passing an electric current through the water.

Paper Whites
Source Institutions
Learners observe different paper samples under ordinary room light and under a black light to learn some of the chemical differences between different types of paper.

Power To Go
Source Institutions
Learners observe an electrochemical cell constructed from a small jar containing zinc and copper strips immersed in separate solutions. The strips are connected to a motor that turns a small fan.

Phase Changes
Source Institutions
Learners observe a sealed test tube containing a small amount of solid stearic acid.

