Search Results
Showing results 1 to 8 of 8

Making a Battery from a Potato
Source Institutions
In this electrochemistry activity, young learners and adult helpers create a battery from a potato to run a clock.
Shocking Fruit
Source Institutions
In this activity, learners discover how a piece of fruit can act as an electrolyte, conducting electricity between two different metals.
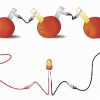
Electricity: Fruit Batteries
Source Institutions
In this activity, learners create a battery from fruit. This activity helps learners explore electricity, electrochemistry, and series circuits as well as the process of scientific inquiry.

Build a Battery
Source Institutions
Learners build a simple one-cell battery and use an ammeter to measure the flow of current.

Kosher Dill Current: Make Your Own Battery!
Source Institutions
This is an activity that demonstrates how batteries work using simple household materials. Learners use a pickle, aluminum foil and a pencil to create an electrical circuit that powers a buzzer.

Can Energy be Created or Destroyed?
Source Institutions
In this activity, learners explore conservation of energy by experimenting with a solar cell light device.

Having a Gas with Water
Source Institutions
In this activity, learners construct a simple electrolysis device. With this device, learners can decompose water into its elemental components: hydrogen and oxygen gas.

Power To Go
Source Institutions
Learners observe an electrochemical cell constructed from a small jar containing zinc and copper strips immersed in separate solutions. The strips are connected to a motor that turns a small fan.
