Search Results
Showing results 1 to 8 of 8

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea
Shocking Fruit
Source Institutions
In this activity, learners discover how a piece of fruit can act as an electrolyte, conducting electricity between two different metals.

Copper Caper
Source Institutions
In this activity, learners conduct an oxidation experiment that turns old pennies bright and shiny. Learners soak 20 dull, dirty pennies in a bowl of salt and vinegar for five minutes.

New Sense about Cents
Source Institutions
In this activity on page 6 of the PDF (Chemistry—It’s Elemental), learners explore some of the properties of copper using a few common household ingredients.
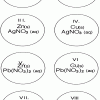
Single Replacement Micro-Reactions
Source Institutions
In this chemistry activity, learners use common chemicals and metals to explore single replacement reactions.

What's in a Penny?
Source Institutions
In this chemistry activity, learners use chemical reactions to observe the composition of an alloy.

A Penny Saved is a Penny Heard
Source Institutions
In this activity (11th activity on the page), learners use pennies to test their hearing acuity.

Penny Battery
Source Institutions
In this activity, learners light an LED with five cents. Learners use two different metals and some sour, salty water to create a cheap battery.
