Search Results
Showing results 1 to 20 of 20

Fold a Crystal
Source Institutions
Rocks are made of minerals, and minerals often have crystal shapes. In this fun activity about geometry in nature, learners create their own crystal shapes out of paper.

Recrystallization Test
Source Institutions
In this activity, learners recrystallize substances from solutions and make observations about the resulting crystals. This test can help further identify the unknown.

Exploring Materials: Liquid Crystals
Source Institutions
In this activity, learners discover that the way a material behaves on the macroscale is affected by its structure on the nanoscale.
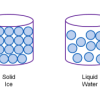
Atoms and Matter (3-6)
Source Institutions
In this activity, learners build models of atoms and molecules, then consider their role in different phases of matter, density, and mixtures and solutions.

The Electric Squeeze
Source Institutions
In this activity/demo about piezoelectricity, learners discover how some crystals produce electricity when squeezed.

Crushing Test
Source Institutions
In this activity, learners design a crushing test and discover that identifying and controlling the variables may be difficult.
Liquid Crystals Interact with Light!
Source Institutions
In this two-part activity, learners explore the properties of liquid crystals, which are responsible for why mood rings change color.
Growing Rock Candy
Source Institutions
In this activity, learners make their own rock candy. Crystals will grow from a piece of string hanging in a cup of sugar water. The edible crystals may take up to a week to form.

Curious Crystals
Source Institutions
Learners carefully look at four known household crystals.

Ice Cream
Source Institutions
In this chemistry activity, learners use the lowered freezing point of water to chill another mixture (ice cream) to the solid state.
Sodium Acetate Hand Warmers
Source Institutions
In this activity, sodium acetate hand warmers are used to introduce learners to supersaturated solutions, crystallization, and exothermic reactions.

Crystal Stencil Stars
Source Institutions
In this activity on page 6 of the PDF, learners dissolve Epsom salt in water and discover that the resulting solution can be used to create a work of art.
Triboluminescence
Source Institutions
In this activity, learners discover what happens when they crush wintergreen-flavored candies in a very dark room.

Crystals: Grow Your Own Garden
Source Institutions
In this simple activity (on page 2 of the PDF), learners make a crystal garden using salt, water, and a brick.

Making Sodium Acetate: Hot Ice
Source Institutions
In this chemistry activity which should only be done under adult supervision (page 10 of the PDF), learners will create an exothermic process by making Sodium Acetate.

Comparing Crystals
Source Institutions
In this chemistry activity (page 3 of the PDF), learners will learn about crystals by growing their very own.

Solubility Test
Source Institutions
In this activity, learners apply a dissolving test to known crystals to identify the unknown. Since the unknown is chemically the same as one of the known crystals, it should dissolve similarly.

Diaper Dissection
Source Institutions
This is written as a display, but can easily be adapted to a hands-on activity. Learners discover how all the parts of a diaper work together to keep babies dry and comfortable.

Supercooled Water Drops
Source Institutions
In this activity, learners touch supercooled water drops with an ice crystal and trigger the water drops to freeze instantly.

Salt Crystal Garden
Source Institutions
In this activity, learners will explore saturated solutions and discover how crystals form.
