Search Results
Showing results 21 to 40 of 65

The Amazing Water Trick
Source Institutions
Using two baby food jars, food coloring, and an index card, you'll 'marry' the jars to see how hot water and cold water mix.

Layered Liquids: Chemistry You Can Drink
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners make a layered drink with liquids of different densities.
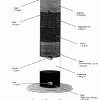
Daffy Density
Source Institutions
In this chemistry activity, learners explore density by using four solids and 6 liquids to create colorful, layered rows.
Floating Golf Ball
Source Institutions
Visitors observe a graduated cylinder with a golf ball floating about halfway in liquid. The bottom half of the cylinder contains a concentrated solution of salt.

Plastics the Second Time Around
Source Institutions
In this activity, learners test and compare the physical properties of thermoplastic polymers. Learners compare different plastics based on their color, degree of transparency, texture, and density.

Fly a Hot-Air Balloon
Source Institutions
Learners assemble a hot-air balloon from tissue paper. The heated air (from a heat gun) inside the balloon is less dense than the surrounding air and causes the balloon to float.

Using Color to See How Liquids Combine
Source Institutions
Learners add different liquids (water, salt water, alcohol, and detergent solution) to water and observe the different ways the different liquids combine with water.

Kimchee Fermentation Chamber
Source Institutions
Learners make kimchee or sauerkraut, which is really just fermented cabbage, in a 2-liter plastic bottle.
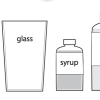
Density Stackers
Source Institutions
In this activity, learners investigate density as they discover how liquids separate to form density layers. Learners discover what happens when they add syrup, cooking oil, and water to a jar.

Bubble Suspension
Source Institutions
In this activity, learners observe as soap bubbles float on a cushion of carbon dioxide gas. Learners blow bubbles into an aquarium filled with a slab of dry ice.

Floating Head Cup
Source Institutions
In this activity, learners watch a figure "magically" float up through the air.

All Mixed Up!
Source Institutions
In this activity, learners separate a mixture of pebbles, salt crystals, and wood pieces. They add water and pour the mixture through a strainer.
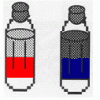
Oil and Soap
Source Institutions
Learners investigate the properties of the liquids in two bottles. One contains layers of oil and water, and one contains oil, water, and soap.

Uplifting Force: Buoyancy & Density
Source Institutions
In this investigation, learners explore the force known as buoyancy by placing various objects into water and observing how they behave (for example, which sink more quickly, which float, how much wat

Dancing Spaghetti
Source Institutions
In this chemistry activity, learners use spaghetti to explore density and chemical reactions.

Challenge: Microgravity
Source Institutions
In this activity about the circulatory system and space travel (on page 38 of the PDF), learners use water balloons to simulate the effects of gravity and microgravity on fluid distribution in the bod
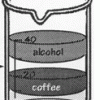
Layers of Liquids
Source Institutions
Learners pour equal amounts of coffee, mineral oil, corn syrup, and alcohol into a beaker. The liquids resolve into stacked layers, and learners can infer which liquids are the most and least dense.

Changing the Density of a Liquid: Heating and Cooling
Source Institutions
Learners investigate how the temperature of water affects its density.

What is in the Water?
Source Institutions
In this activity, learners use open inquiry to learn about the process of science as well as gain experience regarding the Law of Conservation of Mass, dissolution, and density.
Boats Afloat
Source Institutions
In this activity, learners discover what buoyancy is and determine the characteristics that make an object buoyant. Learners design, build, test, and evaluate boats made from a variety of materials.
