Search Results
Showing results 1 to 17 of 17

Fizzy Nano Challenge
Source Institutions
This lesson focuses on how materials behave differently as their surface area increases.

Best Bubbles
Source Institutions
In this activity, learners experiment with creating various types of bubble solutions and testing which ingredients form longer-lasting bubbles.

Why Circulate?
Source Institutions
In this activity related to the human circulatory system (on page 10 of the PDF), learners observe the dispersion of a drop of food coloring in water, draw conclusions about the movement of dissolved

Investigating the Line
Source Institutions
In the related activity called "Colors Collide or Combine," learners are intrigued by the apparent "line" that forms where colors from M&M coatings meet but do not mix.

Biochemistry Happens Inside of You!
Source Institutions
In this four-part activity, learners explore how the body works and the chemistry that happens inside living things.

Curious Crystals
Source Institutions
Learners carefully look at four known household crystals.

M&M's in Different Sugar Solutions
Source Institutions
In this activity, learners investigate whether having sugar already dissolved in water affects the speed of dissolving and the movement of sugar and color through the water.

Matter of Degree
Source Institutions
In two separate bags, learners mix water with Epsom salts and detergent.

Sugar Crystal Challenge
Source Institutions
This lesson focuses on surface area and how the shape of sugar crystals may differ as they are grown from sugars of different coarseness.

What's So Special about Water: Solubility and Density
Source Institutions
In this activity about water solubility and density, learners use critical thinking skills to determine why water can dissolve some things and not others.

Gravestone Weathering
Source Institutions
In this activity (located on pages 9-14 of PDF), learners visit a cemetery to examine the distinguishing characteristics of rock weathering.

Inner Space
Source Institutions
In this activity, learners discover that there is space between molecules even in a cup "full" of water. They first fill a cup with marbles, and then add sand to fill the gaps between the marbles.

Defining Dissolving
Source Institutions
In this introductory activity, learners discover that sugar and food coloring dissolve in water but neither dissolves in oil.
Salting Out
Source Institutions
In this activity, learners create a mixture of water, alcohol and permanent marker ink, and then add salt to form a colored alcohol layer on top of a colorless water layer.

Solubility Test
Source Institutions
In this activity, learners apply a dissolving test to known crystals to identify the unknown. Since the unknown is chemically the same as one of the known crystals, it should dissolve similarly.
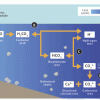
Coral, Carbon Dioxide and Calcification
Source Institutions
In this group activity, learners act out key stages of the "ocean carbon cycle" (also known as the "carbonate buffer system") through motions, rearranging blocks and team tasks.
Cave in a Cup
Source Institutions
In this activity (page 2 of PDF under GPS: Cave Swallows Activity), learners will model how caves are formed by placing one piece of chalk in a cup of vinegar and another piece in a cup of water, then
