Search Results
Showing results 1 to 20 of 25

Matter on the Move
Source Institutions
Learners observe and conduct experiments demonstrating the different properties of hot and cold materials.

Jam Jar Jet
Source Institutions
In this activity, learners create a "Jam Jar Jet" based on Francois Reynst's discovery of a pulsejet engine, which uses one opening for both air intake and exhaust.

Mystery Matter
Source Institutions
This interactive demonstration reintroduces learners to three states of matter (solid, liquid, gas), and introduces them to a fourth state of matter, plasma.
Reaction: Yes or No?
Source Institutions
In this activity, learners mix ingredients in a plastic bag, and then identify three characteristics of a chemical reaction: production of heat, color change, and production of a gas.

A Feast for Yeast
Source Institutions
In this activity on page 6 of the PDF (Get Cooking With Chemistry), learners investigate yeast. Learners prepare an experiment to observe what yeast cells like to eat.

Four of the States of Matter
Source Institutions
This kinesthetic science demonstration introduces learners to four states of matter: solid, liquid, gas, and plasma.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Change in Temperature: Endothermic Reaction
Source Institutions
Learners investigate signs of a chemical reaction when they mix vinegar and baking soda. In addition to a gas being produced, learners also notice the temperature decreases.
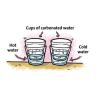
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Air, It's Really There
Source Institutions
This lesson focuses on molecular motion in gases. Learners compare the mass of a basketball when it is deflated and after it has been inflated.

Gas Model
Source Institutions
This highly visual model demonstrates the atomic theory of matter which states that a gas is made up of tiny particles of atoms that are in constant motion, smashing into each other.

Tiny Geyser Models
Source Institutions
In this activity (located on page 2), learners will construct tiny model geysers out of film canisters, warm water, and antacid seltzer tablets.

Avogadro's Bubbly Adventure
Source Institutions
In this activity on page 7 of the PDF, learners investigate the solubility of gas in water at different temperatures. This experiment will help learners determine if temperature affects solubility.

Having a Gas with Water
Source Institutions
In this activity, learners construct a simple electrolysis device. With this device, learners can decompose water into its elemental components: hydrogen and oxygen gas.

Physics by the Fire: Matchstick Rocket
Source Institutions
Learners build a small rocket using a matchstick and a piece of aluminum foil. A second, lit match launches the match rocket. This activity involves fire; adult supervision required.

Sizing Up Temperature
Source Institutions
In this activity, learners explore Charles' Law in a syringe.

Pot-in-Pot Refrigeration
Source Institutions
In this activity (on page 2 of PDF), learners create a low-tech refrigerator that requires no electricity to keep food from spoiling.

What Causes Pressure?
Source Institutions
In this kinesthetic activity that demonstrates pressure, learners act as air molecules in a "container" as defined by a rope.

A Mole of Gas
Source Institutions
In this two-part activity, learners use everyday materials to visualize one mole of gas or 22.4 liters of gas. The first activity involves sublimating dry ice in large garbage bag.

Backyard Biodiesel
Source Institutions
In this activity, learners make a small batch of biodiesel that will work in any diesel engine. Learners use an old juice bottle as a "reactor" vessel to chemically process vegetable oil into fuel.
