Search Results
Showing results 21 to 40 of 47

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).

Candy Chemistry
Source Institutions
In this experiment, learners test multiple food items to see if they are an acid or base using an indicator solution created with red cabbage.

Off Base
Source Institutions
In this activity, learners explore the factors that tend to resist changes in pH of the ocean and why the ocean is becoming more acidic.

Monitoring Amphibians
Source Institutions
In this field study, learners discover how to collect data in the field and how their efforts can help certain animals, specifically, amphibians.

Witches' Potion Demonstration
Source Institutions
In this chemistry demonstration, learners will discover that phenolphthalein is an acid/base indicator. One learner will read a poem about four witches making a potion.

Homework, Hogwarts Style
Source Institutions
In this activity on page 8 of the PDF (Behind the Scenes with Chemistry), learners make three of Harry Potter's essential school supplies: quills, ink, and color-changing paper.

Cabbage Juice Indicator
Source Institutions
In this chemistry activity, learners make indicator solution from red cabbage. Then, learners test everyday foods and household substances using the cabbage juice indicator.

Acids & Bases
Source Institutions
In this activity, learners test the pH of safe liquids available at home by creating a pH indicator from mashed blueberries.

Acid Rain
Source Institutions
In this chemistry demonstration, acid rain is simulated in a petri dish.

Rock Bottoms
Source Institutions
Learners add acid rain (nitric acid) to two cups that represent lakes. One cup contains limestone gravel and the other contains granite gravel.
Stability of Egg White Foams
Source Institutions
In this chemistry meets cooking activity, learners compare the stability of egg white foams with various additives.

Got Gas?
Source Institutions
Create gas with a glass of water, some wire, conductors and a battery! You will be separating water (H2O) into oxygen and hydrogen.
Plastic Milk: You can make plastic from milk
Source Institutions
In this activity (on page 2 of the PDF), learners make a plastic protein polymer from milk. Adding vinegar to milk causes the protein casein to solidify or curdle.
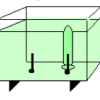
Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.

Cabbage Juice Indicator: Test the pH of household products
Source Institutions
Learners make their own acid-base indicator from red cabbage. They use this indicator to test substances around the house.

Cabbage Patch Chemistry
Source Institutions
In this chemistry activity, learners will learn how to make their own pH indicator using cabbage leaves, and then test common household items with their homemade indicator.

Biotech in a Bag
Source Institutions
In a series of three experiments, learners explore the basics of biotechnology using self-locking plastic baggies. Each experiment demonstrates a phenomenon or principle of biotechnology.

Spicy Indicator: Use turmeric to test for bases in your home
Source Institutions
This activity uses turmeric, a common spice in curry, as an indicator for acidity and basicity. Turmeric is yellow in acid and neutral substances, but turns bright red with bases.

Shell Shifts
Source Institutions
Ocean acidification is a big issue due to the amount of carbon dioxide humans release. CO2 in the atmosphere is absorbed into the ocean thus changing its acidity.

The Proof is in the Powder
Source Institutions
In this activity, learners will design a way to identify a powder found at a crime scene by comparing it with known powders, with the goal of solving a crime.
