Search Results
Showing results 21 to 35 of 35

Dissolving a Substance in Different Liquids
Source Institutions
In this activity, learners make colored sugar and add it to water, alcohol, and oil to discover some interesting differences in dissolving.

Make Your Own Soda Pop
Source Institutions
In this chemistry activity (page 8 of the PDF), learners will identify the instances of physical change, chemical change, and solutions while making homemade soda pop.

What is in the Water?
Source Institutions
In this activity, learners use open inquiry to learn about the process of science as well as gain experience regarding the Law of Conservation of Mass, dissolution, and density.

Comparing Crystals
Source Institutions
In this chemistry activity (page 3 of the PDF), learners will learn about crystals by growing their very own.

Light Soda
Source Institutions
In this activity, learners sublimate dry ice and then taste the carbon dioxide gas.

Disappearing Crystals
Source Institutions
Learners experiment with water gel crystals, or sodium polyacrylate crystals, which absorb hundreds of times their weight in water. When in pure water, the water gel crystals cannot be seen.

Currently Working
Source Institutions
Learners test solutions of water, sugar, salt, and hydrochloric acid for electrical conductivity. They immerse leads from a lighting device (a battery pack connected to an LED) into each solution.

Limewater
Source Institutions
This is a chemistry lab activity about solutions (page 6 of the PDF). Students make a limewater testing solution for carbon dioxide and explore the concepts of solubility and precipitates.

Chemical Breath
Source Institutions
This is a chemistry lab activity about solutions (page 7 of the PDF). Learners see firsthand how chemicals in a solution can combine to form an entirely different substance.

Solubility Test
Source Institutions
In this activity, learners apply a dissolving test to known crystals to identify the unknown. Since the unknown is chemically the same as one of the known crystals, it should dissolve similarly.
Water Wire: Electricity Flowing Through Water
Source Institutions
In this activity on page 10 of the PDF, learners detect the amount of energy that can flow through a sodium chloride electrolyte solution with a light sensor.
Beam Me Up!
Source Institutions
This is a quick activity (on page 2 of the PDF under Stained Glass Activity) about the "Tyndall effect," the scattering of visible light when it hits very small dispersed particles.

Conductivity Meter
Source Institutions
In this activity, learners build a simple qualitative conductivity tester with a battery, bulb and foil.

Having a Gas with Cola
Source Institutions
In this activity, learners measure the amount of carbon dioxide in a carbonated drink.
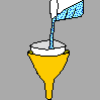
Filtering
Source Institutions
Make a quick and easy filter from household materials. A filter will catch any solids suspended in a liquid and filter them out. By using a filter, learners can discover amazing things.
