Search Results
Showing results 1 to 20 of 80

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea

Separation Anxiety
Source Institutions
In this activity, learners discover the primary physical properties used to separate pure substances from mixtures.

Water Body Salinities I
Source Institutions
In this activity, learners investigate the different salinity levels of oceans, rivers and estuaries.

Watching Crystals Grow
Source Institutions
Learners will compare the growth rate and appearance of crystals forming on small rocks to those growing on miscellaneous objects. Learners will also investigate how temperature (warm vs.

Water Body Salinities II
Source Institutions
In this activity, learners discuss the different salinities of oceans, rivers and estuaries.

Shipping for Survival
Source Institutions
In this activity, learners explore how packaging engineers develop customized shipping and packaging containers to meet the needs of many different industries.

Homemade Hovercraft!
Source Institutions
This activity (on page 2 of the PDF under SciGirls Activity: Hovercraft) is a full inquiry investigation into hovercraft engineering and design optimization.

Composite Materials
Source Institutions
This is an activity (located on page 3 of PDF under Hockey Sticks Activity) about composites, materials made of 2 or more different components.

Mussel Your Way Through Photosynthesis
Source Institutions
Using zebra mussels (Dreissena polymorpha), elodea and an indicator dye, learners study the role of light in photosynthesis.

Head in the Clouds
Source Institutions
In this activity, learners create a CloudSpotter wheel and record the different types of clouds they observe twice daily over several days.

Home Molecular Genetics
Source Institutions
In this activity, learners extract DNA from their own cheek cells, then create a rudimentary DNA profile similar to those seen on crime scene dramas.
Air-filled (Pneumatic) Bone Experiments
Source Institutions
Just like birds, some dinosaurs had air-filled (pneumatic) bones, which made the dinosaurs' skeletons lighter.
Growing Rock Candy
Source Institutions
In this activity, learners make their own rock candy. Crystals will grow from a piece of string hanging in a cup of sugar water. The edible crystals may take up to a week to form.

Introduction to the Scientific Method
Source Institutions
In this activity (page 26 of the PDF), learners make observations, formulate hypotheses and design a controlled experiment, based on the reaction of carbon dioxide with calcium hydroxide.

Cooling Off
Source Institutions
In this activity, learners are introduced to challenges of maintaining temperatures while living in space.

Nano Scavenger Hunt
Source Institutions
This is an activity (located on page 3 of PDF under Where's Nano? Activity) about identifying nanoscale objects and phenomena in today's world.
Making Recycled Paper
Source Institutions
In this activity on page 11 of the PDF, learners follow simple steps to recycle old newspaper into new paper.

Solar Water Heater
Learners work in teams to design and build solar water heating devices that mimic those used in residences to capture energy in the form of solar radiation and convert it to thermal energy.
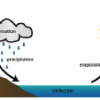
The Water Cycle
Source Institutions
Did you know that the water we use today is the same water found on Earth millions of years ago? The Earth constantly uses and recycles water in a process called the water cycle.

Green Pennies
Source Institutions
In this activity, learners create their own experiment and test which of 4 mixtures of household chemicals turn pennies green over 5 days.
