Search Results
Showing results 1 to 20 of 350

Rainbow Paper
Source Institutions
In this activity, learners will use clear nail polish and the power of chemistry to create paper with a rainbow sheen.

Gravity in Action
Source Institutions
Explore the effects of gravity on a slowly falling object: a parachute you make out of plastic bags, string and stones.

Balloon Isometrics
Source Institutions
In this chemistry activity, learners will explore the concept of entropy. When learners stretch and unstretch a balloon, they will notice a change in temperature.

Exploring Materials: Nano Gold
Source Institutions
In this activity, learners discover that nanoparticles of gold can appear red, orange or even blue. They learn that a material can act differently when it’s nanometer-sized.

Clues About Clouds
Source Institutions
In this weather activity which requires adult supervision, learners will get a chance to make a cloud right here on Earth!
Paper Cover Up
Source Institutions
In this activity on page 11 of the PDF, learners use candle wax to make "invisible" designs that are revealed with watercolor paints.

Mix and Match
Source Institutions
In this activity (7th activity on the page), learners use their sense of hearing to find a "sound match." Learners shake containers filled with items like dry seeds, sand, beans, etc.
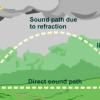
The Rumblin' Road: Determining distance to a Thunderstorm
Source Institutions
In this activity, learners discover how to determine the distance to a lightning strike or nearby thunderstorm.

Density: Make a golf ball float!
Source Institutions
In this activity (on page 2 of the PDF), the learner places a golf ball between salt water and colored fresh water. The golf ball is not as dense as the saltwater.

Exploring Products: Nano Sand
Source Institutions
In this activity, learners explore how water behaves differently when it comes in contact with "nano sand" and regular sand.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.

Chromatography
Source Institutions
In this activity, explore chromatography and the various colors that make up the ink in markers. Use this activity to investigate cohesion and adhesion.
Soda Pop Can Hero Engine
Source Institutions
In this demonstration/activity, water streaming through holes in the bottom of a suspended soda pop can causes the can to rotate.

It's a Gas!
Source Institutions
In this simple activity, learners see the production of a gas, which visibly fills up a balloon placed over the neck of a bottle.

Smelly Balloons
Source Institutions
In this activity, learners sniff out scents hidden in balloons! After investigating, learners discover we sometimes can use another sense (smell) to detect things too small to see.

Exploring Tools: Special Microscopes
Source Institutions
In this activity, learners use a flexible magnet as a model for a scanning probe microscope (SPM). They learn that SPMs are an example of a special tool that scientists use to work on the nanoscale.

Luminol Test
Source Institutions
Learners mix a solution containing luminol and copper with a fake blood solution. A chemical reaction between the luminol solution and fake blood (hydrogen peroxide) show learners a blue glow.

Physics in a Glass: Reversing Arrows
Source Institutions
In this simple activity, learners investigate refraction by placing a picture of an arrow behind a glass of water.
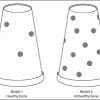
Space Stations: Bones of Contention
Source Institutions
In this activity, learners make models representing bones on Earth and bones that have been in space. They discover what happens to bones without proper exercise and nutrition.

Clear Slime Polymer
Source Institutions
In this chemistry activity, learners use guar gum to make slime. Use this activity to introduce learners to polymers, viscosity, and colloids.
