Search Results
Showing results 1 to 20 of 39

Balloon Isometrics
Source Institutions
In this chemistry activity, learners will explore the concept of entropy. When learners stretch and unstretch a balloon, they will notice a change in temperature.

Clues About Clouds
Source Institutions
In this weather activity which requires adult supervision, learners will get a chance to make a cloud right here on Earth!
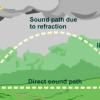
The Rumblin' Road: Determining distance to a Thunderstorm
Source Institutions
In this activity, learners discover how to determine the distance to a lightning strike or nearby thunderstorm.
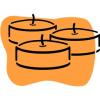
Floating Candles
Source Institutions
In this chemistry activity, learners observe a combustion reaction and deduce the components necessary for the reaction to occur.

Rate of Solution Demonstration
Source Institutions
In this chemistry demonstration, learners investigate the factors that increase the rate of dissolution for a solid.

Mystery Matter
Source Institutions
This interactive demonstration reintroduces learners to three states of matter (solid, liquid, gas), and introduces them to a fourth state of matter, plasma.

Solar Convection
Source Institutions
In this activity, learners add food coloring to hot and cold water in order to see how fluids at different temperatures move around in convection currents.

The Amazing Water Trick
Source Institutions
Using two baby food jars, food coloring, and an index card, you'll 'marry' the jars to see how hot water and cold water mix.

Meltdown
Source Institutions
In this activity, learners heat ice and water of the same temperature to get a hands-on look at phase changes. This is an easy and inexpensive way to introduce states of matter and thermodynamics.

Bake Ice Cream in Your Oven
Source Institutions
In this a hands-on activity, learners explore how to put ice cream in an oven without it melting. Ideas in this activity include insulation and cooking.

That Sinking Feeling
Source Institutions
In this quick activity, learners observe how salinity and temperature affect the density of water, to better understand the Great Ocean Conveyor.
How Animals Stay Warm
Source Institutions
In this quick activity, learners explore how blubber protects animals from the cold by making a "blubber mitt." Using cooking shortening, two zip-top sandwich bags, and duct tape, learners simulate bl

Can Crushers
Source Institutions
In this activity, learners conduct an experiment by heating an aluminum can filled with water to investigate air pressure.
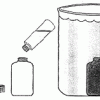
Make Your Own Deep-Sea Vent
Source Institutions
In this activity, learners make a model of the hot water of a deep sea vent in the cold water of the ocean to learn about one of the ocean's most amazing and bizarre underwater habitats.

Breaking Up with Combustion
Source Institutions
This activity teaches combustion as the interaction of a fuel source and oxygen.

Aerogel
Source Institutions
This activity/demo introduces learners to aerogel, a glass nanofoam. Learners discover how aerogel is made and how well it insulates as well as learn about aerogel's other unique properties.

What is a “Convection Cell”?
Source Institutions
In this demonstration, learners can observe a number of small convection cells generated from a mixture of aluminum powder and silicon oil on a hot plate.

Air Pressure
Source Institutions
In this experiment, learners use a blow dryer and water bottle to observe and record changes in air pressure caused by changes in temperature.

Do Sweat It!
Source Institutions
In this activity, learners explore why humans sweat. Learners compare the effects of heat on a balloon filled with air and a balloon filled water.
Physical Change
Source Institutions
In this activity, learners use heat to separate zinc and copper in a penny. This experiment demonstrates physical properties and how physical change (phase change) can be used to separate matter.
