Search Results
Showing results 21 to 40 of 156

Natural Buffers
Source Institutions
Learners use a universal indicator to test the amount of sodium hydroxide needed to change the pH of plain water compared with the amount needed to change the pH of gelatin.
Egg Osmosis
Source Institutions
Visitors observe three beakers. One beaker contains an egg immersed in vinegar. Visitors observe carbon dioxide gas escaping from the shell as the calcium carbonate reacts with the vinegar.

Slinky in Hand: Make waves without getting wet
Source Institutions
Play with a slinky and make transverse waves. In this simple Exploratorium Science Snack, learners will experience making waves and will learn the different parts of a wave.

Atmospheric Collisions
Source Institutions
In this activity/demonstration, learners observe what happens when two ping pong balls are suspended in the air by a hair dryer. Use this activity to demonstrate how rain drops grow by coalescence.

Red, White and Blue II Demonstration
Source Institutions
In this chemistry demonstration, learners investigate the rule "likes dissolve likes" by combining three, immiscible liquids to create a colorful density column.

Open Heart: Disease and Diagnosis
Source Institutions
In this online activity about heart disease, learners will pick one of three patients (based on actual patient histories) and help a cardiologist diagnose and prescribe treatments for him or her.

Loony Balloons
Source Institutions
In this activity, learners investigate how changing the center of gravity of a balloon affects how it travels. Learners fill a balloon with a little bit of water and insert into an empty balloon.
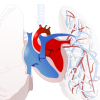
Construct a Heart Circulation Model
Source Institutions
In this online activity about anatomy, learners will correctly order the path blood takes through the heart in order to absorb oxygen to deliver to the rest of the body.

Magical Match
Source Institutions
In this demonstration, learners will be "wowed" as three matches burn to form a triangular pyramid shape and "magically" rise off the table.
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.

Hot and Cold
Source Institutions
In this activity, learners explore temperature changes from chemical reactions by mixing urea with water in one flask and mixing calcium chloride with water in another flask.

Red, White and Blue I Demonstration
Source Institutions
In this chemistry demonstration, learners observe a chemical reaction that produces a colorful effect.

One In The Hand
Source Institutions
In this physics demonstration, learners are challenged to break a raw egg just by squeezing it. Learners will be shocked by their inability to complete the deceivingly simple challenge.
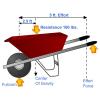
Find the Simple Machines
Source Institutions
This is a web activity about simple machines. Learners will explore a lawn mower and identify six different simple machines which work together to help make our lives easier.

Egg-Citing Physics
Source Institutions
In this demonstration about momentum, use physics to distinguish between a hard-boiled egg and a raw egg without cracking them open.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.

Go with the Flow
Source Institutions
In this activity, learners discover how hard their hearts work to pump blood.
Are you a Supertaster?
Source Institutions
In this activity, learners examine their tongue and taste buds.
