Search Results
Showing results 161 to 180 of 264

Kinetic Sculpture
Source Institutions
In this design challenge activity, learners build a tower that’s at least 12 inches high with two or more parts that move (spin, sway, or flap) in the wind.

Explore Like a Scientist: Fruity Observations
Source Institutions
In this activity, learners use their senses to collect information about a fruit and record their findings in a Science Journal.

“Chips” Off the Old Block?
Source Institutions
In this activity, learners bake cookies by following different recipes to better understand genetic mutations. Everyone in the group bakes the same type of cookie: chocolate chip.

Rescue Rover
Source Institutions
In this activity, learners explore how rescue devices are designed to aid professionals during emergency situations.

Gas Model
Source Institutions
This highly visual model demonstrates the atomic theory of matter which states that a gas is made up of tiny particles of atoms that are in constant motion, smashing into each other.

Marine Skulls Cart
Source Institutions
In this activity, learners look at and touch marine animal skulls to compare them and think about what they eat.

Dance Pad Mania
Source Institutions
Make your own "Dance Dance Revolution" dance pad! In this design challenge activity, learners work in teams to build a dance pad that lets you use your feet to sound a buzzer or flash a light.

Tree Growth Investigation
Source Institutions
This activity (located on page 3 of the PDF under GPS: Temperate Rain Forest Activity) is a full inquiry investigation into tree growth rates.

Science of Sunblock
Source Institutions
This is an activity (located on page 3 of the PDF under Stained Glass Activity) about nanotechnology making its way into everyday products, such as sunscreen, and how effective these invisible particl
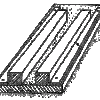
Clay Beams and Columns
Source Institutions
In this activity, learners make or use pre-made clay beams to scale and proportion. Specifically, they discover that when you scale up proportionally (i.e.

Can You Copperplate?
Source Institutions
In this activity, learners explore chemical engineering and how the processes of chemical plating and electroplating have impacted many industries.

Pulleys and Force
Source Institutions
In this activity, learners explore the concept of force and how pulleys are used in everyday life to make work easier.
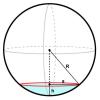
Mass, Area, Volume
Source Institutions
In this activity (page 18 of PDF), learners will measure the volume of impact craters created by projectiles of different masses.

Cast Your Vote
Source Institutions
In this activity, learners explore how voting systems have changed with engineering advances over time. Learners work in teams to design their own voting system using easy to find materials.

Pipeline Challenge
Source Institutions
In this activity, learners explore how engineers develop pipeline systems to transport oil, water, gas, and other materials over very long distances.

Fossil Dig Site
Source Institutions
In this activity (located on page 5 of PDF), learners work in groups to create dig sites for display.

Water Fountain
Source Institutions
In this activity, learners explore how a hydraulic pump works. Learners work in teams to design and build a unique water fountain that employs a hydraulic pump.

Tennis Anyone?
Source Institutions
In this activity, learners explore sports engineering and advanced materials development.

Build a Coral Polyp
Source Institutions
In this activity, learners build one or more edible coral polyps and place them together to form a colony.

EEEEK--A Mouse!
Source Institutions
In this activity, learners explore the concept of how engineering solved the problem of human/computer interface.
