Search Results
Showing results 121 to 140 of 172

Whodunit?
Source Institutions
In this fascinating and fun experiment, learners use chemistry to identify a mystery powder and to solve a "crime," a process similar to that used by real forensic scientists.

Ocean Home: Swimming Fishes
Source Institutions
In this activity, learners model, on a human-sized board game, how changes in water temperature may affect fish distributions and, ultimately, fisheries.
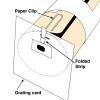
Spectroscope
Source Institutions
In this activity, learners construct their own spectroscope as they explore and observe spectra from familiar light sources.

Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.

Cool It!
Source Institutions
Learners make a refrigerator that works without electricity. The pot-in-pot refrigerator works by evaporation: a layer of sand is placed between two terra cotta pots and thoroughly soaked with water.

Wintergreen
Source Institutions
In this outdoor, winter activity, learners find living green plants under the snow and determine the light and temperature conditions around the plants.

Microbes are Everywhere
Source Institutions
In this four-day activity, learners grow bacteria and/or fungi from a variety of locations and compare the results.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Currently Working
Source Institutions
Learners test solutions of water, sugar, salt, and hydrochloric acid for electrical conductivity. They immerse leads from a lighting device (a battery pack connected to an LED) into each solution.

Macro-Microarray
Source Institutions
In this activity, learners explore the "nuts and bolts" of gene chips.

Sand Activity
Source Institutions
In this activity, learners observe mixtures of sand samples glued to note cards, and consider how sand can differ in size, shape, and color, and where it comes from.

Bacteriopolis
Source Institutions
In this long-term activity, learners make a home for a colorful community of microorganisms.
Take Out the Trash
Source Institutions
Learners explore how recyclers take advantage of the different properties of materials, such as magnetism and density, to separate them from a mixture.

Jay Play
Source Institutions
In this outdoor activity, learners find out the color of food that jays prefer and then try to change the birds' preference by altering the taste of the food with salt.

Clam Hooping
Source Institutions
In this two-part outdoor activity, learners conduct a population census of squirting clams on a beach or mudflat, and investigate the clams' natural history.

Mystery of the Disappearing Cottonwoods
Source Institutions
Learners will explore the scientific mystery behind a disappearing group of trees by examining data and attempting to explain the decline.

Fireworks!
Source Institutions
In this chemistry lab activity, learners model the colors of fireworks by burning metallic solutions in a flame and observing the different colors produced.

Sky Time: Kinesthetic Astronomy
Source Institutions
Through a series of simple body movements, learners gain insight into the relationship between time and astronomical motions of Earth (rotation about its axis, and orbit around the Sun), and also abou

Wild Sourdough
Source Institutions
In this activity, learners explore chemistry and the microbial world by making their own sourdough starter and bread at home using only flour and water.

Jelly Beads
Source Institutions
Learners add drops of alginate solution to a solution of calcium chloride. The alginate does not mix with the calcium chloride, but forms soft gel beads.
