Search Results
Showing results 1 to 19 of 19

Cleaning with Dirt
Source Institutions
Learners build a filter from old soda bottles and dirt. They create polluted water, and pour it through their filter to clean it.
Trading Places: Redox Reactions
Source Institutions
Visitors add drops of copper sulfate solution onto a steel nail. They observe the nail change color from silver to brown as the copper plates onto the nail.
Currently Working: Testing Conductivity
Source Institutions
Visitors test solutions of water, sugar, salt, and hydrochloric acid and the solids salt and sugar. They clip leads from the hand generator to wires immersed in each substance.
Forwards and Backwards: pH and Indicators
Source Institutions
Visitors prepare six solutions combining vinegar and ammonia that range incrementally from acid (all vinegar) to base (all ammonia).
Hot and Cold: Endothermic and Exothermic Reactions
Source Institutions
Visitors mix urea with water in one flask and mix calcium chloride with water in another flask. They observe that the urea flask gets cold and the calcium chloride flask gets hot.
Yeast Balloons
Source Institutions
Visitors observe a bottle with a balloon attached around the mouth. The bottle contains a solution of yeast, sugar, and water.
It's A Gas!
Source Institutions
Visitors mix water and sodium bicarbonate (baking soda) in a large flask. They then add citric acid to the mixture and stopper the flask. The resulting reaction creates carbon dioxide gas.
All Mixed Up!: Separating Mixtures
Source Institutions
Visitors separate a mixture of pebbles, salt crystals, and wood shavings by adding water and pouring the mixture through a strainer.
Shrinkers
Source Institutions
Visitors use heat to shrink samples of polystyrene. They compare samples from containers that were shaped in different ways during manufacturing.
Concentrate: Concentrations and Reaction Rates
Source Institutions
Visitors incrementally increase the amount of iodate in three different test tubes containing the same amount of a starch solution.

Electroplating
Source Institutions
In this activity, learners electrically plate zinc onto brass objects.

A Hole in the Ground
Source Institutions
Learners build models of sinkholes to gain an intuitive knowledge of their physical aspects.
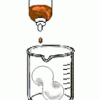
Foam Peanuts
Source Institutions
Learners compare the properties and solubilities of Styrofoam (TM), ecofoam packing peanuts, and popcorn. First, the solubility of each substance is tested in water.
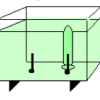
Electrolysis
Source Institutions
Using electrolysis, learners produce hydrogen gas and oxygen gas from water molecules in a solution.

Cool It!
Source Institutions
Learners make a refrigerator that works without electricity. The pot-in-pot refrigerator works by evaporation: a layer of sand is placed between two terra cotta pots and thoroughly soaked with water.

Mystery of the Disappearing Cottonwoods
Source Institutions
Learners will explore the scientific mystery behind a disappearing group of trees by examining data and attempting to explain the decline.

Magical Möbius
Source Institutions
In this tabletop activity (on pages 32-40), learners make Möbius strips -- 3D surfaces with only one side.

Paperfolding Polyhedrons
Source Institutions
In this activity (on pages 55-66 of PDF), learners fold paper into origami shapes and then combine several identical shapes into a three-dimensional structure.

Gross Growth
Source Institutions
In this activity, learners grow germs collected from their hands and other objects. They cultivate the germs on a growth medium (such as slices of grapefruit or processed cheese) for a week.
