Search Results
Showing results 21 to 33 of 33

Using Color to See How Liquids Combine
Source Institutions
Learners add different liquids (water, salt water, alcohol, and detergent solution) to water and observe the different ways the different liquids combine with water.

Spaghetti Strength
Source Institutions
In this activity on page 7 of the PDF, learners explore how engineers characterize building materials.

Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Developing Tests to Distinguish Between Similar-Looking Unknowns
Source Institutions
Learners identify an unknown liquid by comparing its behavior to known liquids. Learners drop liquids onto different surfaces and see how the liquids behave.

Changing the Density of a Liquid: Heating and Cooling
Source Institutions
Learners investigate how the temperature of water affects its density.
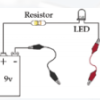
Exploring Materials: Graphene
Source Institutions
In this activity on page 4 of the PDF, explore the unique molecular structure and conductive nature of graphene. Learners construct a circuit with a battery and LED bulb.

Changing the Density of an Object: Adding Material
Source Institutions
Learners see that a can of regular cola sinks while a can of diet cola floats. As a demonstration, bubble wrap is taped to the can of regular cola to make it float.

Production of a Gas: Controlling a Chemical Reaction
Source Institutions
Learners mix vinegar and baking soda to produce a gas. With the addition of a bit of liquid soap, the gas becomes trapped in measurable bubbles.

From Gas to Liquid to Solid
Source Institutions
What causes frost to form on the outside of a cold container? In this activity, learners discover that liquid water can change states and freeze to become ice.

Solubility Test
Source Institutions
In this activity, learners apply a dissolving test to known crystals to identify the unknown. Since the unknown is chemically the same as one of the known crystals, it should dissolve similarly.

Comparing the Density of Different Liquids
Source Institutions
Learners carefully pour vegetable oil, water, and corn syrup in any order into a cup and discover that regardless of the order they are poured, the liquids arrange themselves in layers the same way.

Snow Day!
Source Institutions
In this activity (on pages 4-5), learners make fake snow by adding water to the super-absorbant chemical from diapers, sodium polyacrylate.

Change in Temperature: Exothermic Reaction
Source Institutions
Learners add calcium chloride to a baking soda solution and observe an increase in temperature along with the production of a gas and a white precipitate. These are all signs of a chemical reaction.
