Search Results
Showing results 441 to 460 of 872

How does the Atmosphere keep the Earth Warmer?
Source Institutions
In this activity, learners simulate the energy transfer between the earth and space by using the light from a desk lamp desk lamp with an incandescent bulb and a stack of glass plates.

Exploring A Hydrogel
Source Institutions
In this activity on page 10 of the PDF, learners develop an experiment to answer the following question: "How much water can the hydrogel in a baby diaper hold?" Use this activity to explore polymers,

The Wave
Source Institutions
In this multi-step experiment (page 4 of pdf), learners study tide pools, and then drop a "wave" (a 4-pound bag of beans or birdseed) on a shell to mimic the force of crashing surf on tide pool animal

Water Cycle in a Bag
Source Institutions
In this activity, learners create a biosphere in a baggie.

What Causes Wind?
Source Institutions
In this sunny day experiment, learners measure and compare how quickly light and dark colored materials absorb heat.

Living Clocks
Source Institutions
In this activity about daily rhythms (on page 17 of the PDF), learners will explore circadian patterns in humans, animals and plants.

Try Your Hand at Nano
Source Institutions
This lesson focuses on two simple activities that younger learners can do to gain an appreciation of nanotechnology. First, learners measure their hands in nanometers.
Muscle Fibers
Source Institutions
In this activity about human anatomy (page 20 of PDF), learners investigate the structure of muscles by comparing yarn and cooked meat.
Do Plants Need Light?
Source Institutions
In this food science activity, learners conduct an experiment that demonstrates the importance of light to plants.

Dancing Spaghetti
Source Institutions
In this chemistry activity, learners use spaghetti to explore density and chemical reactions.

Production of Oxygen
Source Institutions
In this chemistry activity, learners use yeast and hydrogen peroxide to generate a gas (oxygen) and test some of its properties.

Make Your Own Batteries!
Source Institutions
This activity (on page 3 of the PDF under GPS: Body Electricity Activity) is a full inquiry investigation into conductivity.

New Sense about Cents
Source Institutions
In this activity on page 6 of the PDF (Chemistry—It’s Elemental), learners explore some of the properties of copper using a few common household ingredients.

Paper Drop Design Competition
Source Institutions
Using paper, paper clips, an index card, and tape, teams of learners design flying devices to (1) stay in the air as long as possible and (2) land as close as possible to a given target.

Cleaning Water with Dirt
Source Institutions
In this activity on page 7 of the PDF (Water in Our World), learners make their own water treatment systems for cleaning water.

Testing Vitamin C: Chemistry's Clear Solution
Source Institutions
In this activity on page 8 of the PDF, learners investigate vitamin C. Learners conduct a chemistry experiment to determine if Tang drink mix or orange juice contains more vitamin C.

Forwards and Backwards
Source Institutions
In this activity, learners explore acids and bases by preparing six solutions that combine vinegar and ammonia, ranging from acid (all vinegar) to base (all ammonia).

Production of Hydrogen
Source Institutions
In this chemistry activity, learners use mossy zinc (or a galvanized nail) and hydrochloric acid to generate hydrogen gas and test some of its properties.

Combustion
Source Institutions
In this chemistry activity, learners discover that the weight of the product of combustion is greater than that of the starting material.
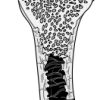
Round, Light and Hollow
Source Institutions
In this activity about bones (page 12 of PDF), learners investigate and compare the weight-bearing capacity of solid and hollow cylinders.
