Search Results
Showing results 301 to 320 of 978

Dinosaur Breath
Through discussion and hands-on experimentation, learners examine the geological (ancient) carbon cycle.

Nano Waterproofing
Source Institutions
This lesson focuses on how nanotechnology has impacted the design and engineering of many everyday items from paint to fabrics.

Law of Conservation of Mass
Source Institutions
In this chemistry activity, learners explore whether matter is created or destroyed during a chemical reaction. They will compare the weight of various solutions before and after they are mixed.
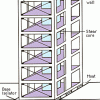
Earthquake-Proof Buildings
Source Institutions
In this geology and engineering activity (page 7 of the PDF), learners build an earthquake simulator, then use it to test various building designs, exploring different materials, shapes, and design op

Kosher Dill Current: Make Your Own Battery!
Source Institutions
This is an activity that demonstrates how batteries work using simple household materials. Learners use a pickle, aluminum foil and a pencil to create an electrical circuit that powers a buzzer.

How Long Can You Hold Your Breath?
Source Institutions
In this activity (on page 142 of the PDF), learners will compare breathing rates before and after hyperventilation to explore how reduced carbon dioxide levels in the blood lower the need to breathe.

Of Cabbages and Kings
Source Institutions
This lesson gives full instructions for making cabbage juice indicator, a procedure sheet for learners to record observations as they use the indicator to test materials, and extension activities to d

Trading Places
Source Institutions
In this activity, learners discover that atoms and ions of different metals will change places.
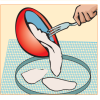
Making Recycled Paper
Source Institutions
In this activity on page 11 of the PDF, learners follow simple steps to recycle old newspaper into new paper.

Racing M&M Colors
Source Institutions
Learners design their own experiment to determine which M&M color dissolves the fastest in water.

Hover Cup
Source Institutions
Is this activity concentrating on physical science, learners build their very own miniature hovercraft out of a paper cup. Using it, they can explore the concepts of friction and force.

Invisible Sunblock
Source Institutions
This is a hands-on activity exploring how nanoscale particles are used in mineral sunblocks to increase their transparency.

Fizzy Fun
Source Institutions
In this activity, learners test what happens when they put baking power on different frozen liquids.

Exploring Moisture on the Outside of a Cold Cup
Source Institutions
In this activity, learners explore the relationship between cooling water vapor and condensation. Learners investigate condensation forming on the outside of a cold cup.

Light is Made of Colors
Source Institutions
Learners observe different light sources, outdoors and indoors, using prism glasses (diffraction glasses) and color filters.

Wheat Germ DNA Extraction
Source Institutions
This laboratory exercise is designed to show learners how DNA can easily be extracted from wheat germ using simple materials.

Leaf it to Me
Source Institutions
In this activity, learners observe the effect of transpiration as water is moved from the ground to the atmosphere.

Name That Frequency
Source Institutions
This activity was designed for blind learners, but all types of learners can model how vibrating particles, such as in a sound wave, bump into other particles causing them to vibrate, and that the vib

A Simply Fruity DNA Extraction
Source Institutions
In this activity, learners extract DNA from a strawberry and discover that DNA is in the food they eat.

