Search Results
Showing results 1041 to 1060 of 1185

Shell Shifts
Source Institutions
Ocean acidification is a big issue due to the amount of carbon dioxide humans release. CO2 in the atmosphere is absorbed into the ocean thus changing its acidity.

Plant Tissue Culture: Classroom Activities in Plant Biotechnology
Source Institutions
In this activity related to plant biotechnology, learners use the tissue culture process to rapidly produce clones (genetic copies) of a particular plant (cauliflower, rose cuttings, African violet le

A Swell Activity with Beans
Source Institutions
In this combination chemistry and physics activity, learners explore water absorption in dried beans or peas and learn how this affects their physical properties.

Gel Electrophoresis
Source Institutions
In this activity, learners simulate the process of DNA fingerprinting by using electricity to separate colored dyes.

What is Light?
Source Institutions
In this four-part activity, learners will discover the exciting world of light--the most important form of energy in our world--and be able to identify and describe different types of light.

Plaster of Paris
Source Institutions
In this activity (page 6 of the PDF), learners will observe both a chemical and a physical change.

Colors, Colors?
Source Institutions
In this activity related to the famous "Stroop Effect," learners explore how words influence what we see and how the brain handles "mixed messages." Learners read colored words and are asked to say th

Our Sense of Hearing
Source Institutions
In this activity, learners investigate the sense of hearing and plan and conduct their own experiments.
Poking Around: Having Students Experience the Real Process of Science
Source Institutions
Using indirect methods, learners determine the shape and size of a piece of carpet hidden under a piece of plywood.
Geometry and Algebra: The Future Flight Equation
Source Institutions
In this activity, learners discover how NASA engineers develop experimental aircraft.

Bone Identification
Source Institutions
This activity (page 3 of the PDF under SciGirls Activity: Dinosaurs) is a full inquiry investigation into fossil hunting and identification.

Does Sunscreen Protect My DNA?
Source Institutions
In this laboratory experiment, learners explore how effectively different sunscreens protect yeast cells from damage caused by ultraviolet (UV) radiation.

Newton's 2nd Law: Inquiry Approach
Source Institutions
In this lab activity, learners act as fellow scientists and colleagues of Isaac Newton. He has asked them to independently test his ideas on the nature of motion, in particular his 2nd Law.

The Proof is in the Powder
Source Institutions
In this activity, learners will design a way to identify a powder found at a crime scene by comparing it with known powders, with the goal of solving a crime.

Density Stacker
Source Institutions
In this physics activity (page 8 of the PDF), learners will explore the property of density.

Checking For Starch
Source Institutions
In this chemistry activity (page 3 of the PDF), learners will observe a chemical change, specifically what happens to iodine when it is applied to ripe and unripe apples.

Our Chemical Senses: Olfaction
Source Institutions
In this activity, learners investigate the olfactory system by conducting several experiments.
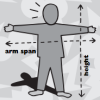
Are you a Square or a Rectangle?
Source Institutions
In this activity, learners investigate whether more people are squares or rectangles. People with similarly sized heights and arm spans are classified as squares.

Clothespin Workout
Source Institutions
This is a great activity about human energy production. Learners will work out with a clothespin to investigate why hockey players jump on a stationary bike after an intense game.
Water Filter
Source Institutions
In this engineering activity, challenge learners to invent a water filter that cleans dirty water.
