Search Results
Showing results 181 to 200 of 799
The Nose Knows!
Source Institutions
In this activity on page 9 of the PDF, learners test how flavoring extracts move through the walls of a balloon.
Floating Paperclip and Other Surface Tension Experiments
Source Institutions
In this activity, learners experiment with surface tension using everyday household items such as strawberry baskets, paperclips, liquid dish soap, and pepper.
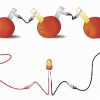
Electricity: Fruit Batteries
Source Institutions
In this activity, learners create a battery from fruit. This activity helps learners explore electricity, electrochemistry, and series circuits as well as the process of scientific inquiry.

Indicating Electrolysis
Source Institutions
In this activity, learners build a simple electrolysis device. Then learners use an indicating solution to visualize hydrogen and oxygen molecules in water.

Introduction to the Scientific Method
Source Institutions
In this activity (page 26 of the PDF), learners make observations, formulate hypotheses and design a controlled experiment, based on the reaction of carbon dioxide with calcium hydroxide.

Density Rainbow
Source Institutions
In this activity, learners mix several sugar solutions to investigate the property of density. Each sugar solution has a different density and color of the rainbow.

pHun with Cabbage
Source Institutions
In this chemistry activity, learners will test the pH of various foods and household substances using cabbage.
Neural Network Signals
Source Institutions
In this activity, learners create an electrical circuit and investigate how some dissolved substances conduct electricity.

Hot Stuff!: Testing for Carbon Dioxide from Our Own Breath
Learners blow into balloons and collect their breath--carbon dioxide gas (CO2). They then blow the CO2 from the balloon into a solution of acid-base indicator.
Spill Spread
Source Institutions
In this simulation, learners explore how ocean currents spread all kinds of pollution—including oil spills, sewage, pesticides and factory waste—far beyond where the pollution originates.

Experimenting with Naked Eggs
Source Institutions
In this activity about osmosis, learners use a naked egg (one with a dissolved eggshell) to learn about selectively permeable membranes.

Radial Chromatography
Source Institutions
How many colors make black? Gather as many water soluble black markers as you can find.

A Dissolving Challenge
Source Institutions
In this activity, learners add objects and substances to carbonated water to discover that added objects increase the rate at which dissolved gas comes out of solution.

Wash This Way
Source Institutions
In this activity on page 4 of the PDF, learners investigate the importance of washing their hands.

Water: Clearly Unique!
Source Institutions
In this activity on page 4 of the PDF (Water in Our World), learners conduct some quick and easy tests to determine the differences between water and other liquids that look very similar to water.

Build a Battery
Source Institutions
Learners build a simple one-cell battery and use an ammeter to measure the flow of current.

Fuel for Living Things
Source Institutions
In this activity, learners observe what happens when yeast cells are provided with a source of food (sugar). Red cabbage "juice" will serve as an indicator for the presence of carbon dioxide.

Ziptop Bag Chemistry
Source Institutions
In this chemistry activity, learners perform three chemical reactions in a sealed zip-top bag. Learners will record their observations and classify the changes as chemical or physical.
Pathways with Friends
Source Institutions
Directed by instructional cards, learners kinesthetically model cell communication by acting as components in a cell signaling pathway.

Layered Liquids: Chemistry You Can Drink
Source Institutions
In this chemistry activity (on page 2 of the PDF), learners make a layered drink with liquids of different densities.
