Search Results
Showing results 581 to 600 of 799

To Dye For
Source Institutions
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each.

Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.

Squidgy Slime
Source Institutions
In this chemistry activity, learners transform two ingredients (4% polyvinyl alcohol solution and 4% borax solution) into gooey slime.

Sticky Engineering Challenge
Source Institutions
In this activity, learners explore how engineers work in a team to solve problems.

Iron in Cereal: Find iron in your food!
Source Institutions
Learners investigate an iron-fortified cereal by stirring it with a strong magnet. They discover that metallic iron is present in some cereals.
As Light as Air
Source Institutions
Learners measure a bottle full of air, and then use a vacuum pump to remove the air. When they re-weigh the bottle, learners find the mass is about 0.8g less.

Milk Plastic
Source Institutions
In this activity, learners transform everyday milk into small plastic figurines and jewelry. Use this activity to introduce learners to monomers and polymers.

Photosynthesis and Transpiration
Source Institutions
In this activity on page 7 of the PDF (Plants—The Green Machines), learners examine the effects that light and air have on green plants.

Shake the Bag Ice Cream
Source Institutions
In this activity, learners will experiment with salt and ice in order to turn a bag of ingredients into ice cream.

Levity Through Tension: Fun with Water's Surface Tension
Source Institutions
This experiment describes how to create a "dribble bottle" which only leaks water when the cap is unscrewed. The full water bottle has a small hole made with a push pin.

Cabbage Patch Chemistry
Source Institutions
In this chemistry activity, learners will learn how to make their own pH indicator using cabbage leaves, and then test common household items with their homemade indicator.

Chemistry Makes Scents
Source Institutions
In "Chemistry Makes Scents," participants use their noses to distinguish between chemicals with very similar structures.

Pages of a Forbidden Tome
Source Institutions
In this activity, learners use chemistry to produce weathered "antiqued" paper with burned edges. Learners first soak white paper in coffee and then apply a charring solution of ammonium chloride.

Having a Gas with Water
Source Institutions
In this activity, learners construct a simple electrolysis device. With this device, learners can decompose water into its elemental components: hydrogen and oxygen gas.
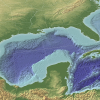
The Dead Zone: A Marine Horror Story
Source Institutions
In this environmental science and data analysis activity, learners work in groups to track a Dead Zone (decreased dissolved oxygen content of a body of water) using water quality data from the Nutrien

Biotech in a Bag
Source Institutions
In a series of three experiments, learners explore the basics of biotechnology using self-locking plastic baggies. Each experiment demonstrates a phenomenon or principle of biotechnology.
Choose Your Ooze
Source Institutions
During this activity, learners will make different versions of "ooze" using varied proportions of detergent and glue.

Miscibility
Source Institutions
Learners observe a bottle containing water and oil. They are invited to pick up the bottle and mix the contents together.

Potato Power
Source Institutions
Learners combine hydrogen peroxide with three different forms of potato: raw chunks, ground chunks, and boiled chunks.

Energy Sources
Source Institutions
In this activity about the relationship between food and energy (page 5 of PDF), learners conduct an experiment to compare how much energy is released as heat from two different foods.
