Search Results
Showing results 1 to 20 of 155

Cleaning with Dirt
Source Institutions
Learners build a filter from old soda bottles and dirt. They create polluted water, and pour it through their filter to clean it.

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea

Acid (and Base) Rainbows
Learners use red cabbage juice and pH indicator paper to test the acidity and basicity of household materials. The activity links this concept of acids and bases to acid rain and other pollutants.
Mercury in the Environment
Source Institutions
In this environmental science lesson, learners will examine the dangers of mercury and how humans contribute to growing mercury emissions on Earth.
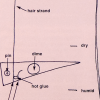
Better Hair Through Chemistry
Source Institutions
In this activity, learners hook up a hair to a lever system and create a hair hygrometer to measure changes in humidity.

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

Salt 'n Lighter
Source Institutions
In this activity, learners discover that as the salinity of water increases, the density increases as well. Learners prove this by attempting to float fresh eggs in saltwater and freshwater.

Water Body Salinities I
Source Institutions
In this activity, learners investigate the different salinity levels of oceans, rivers and estuaries.
Investigating Density Currents
Source Institutions
In this lab activity, learners explore how to initiate a density current. Learners measure six flasks with different concentrations of salt and water (colored blue).

Fragile Waters
Source Institutions
In this activity (on pages 18-29) learners explore the impact of the March 24, 1989 oil spill in Alaska caused by the Exxon Valdez tanker.

Watching Crystals Grow
Source Institutions
Learners will compare the growth rate and appearance of crystals forming on small rocks to those growing on miscellaneous objects. Learners will also investigate how temperature (warm vs.

Surface Tension Icebreaker
Source Institutions
This is a quick activity (located on page 2 of the PDF under Nasturtium Leaves Activity) about surface tension.
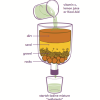
Water Clean-up
Source Institutions
This is an activity (located on page 3 of the PDF under Water Clean-up Activity) about the use of reduction agents to decontaminate ground water.

Water Body Salinities II
Source Institutions
In this activity, learners discuss the different salinities of oceans, rivers and estuaries.

Diet Light
Source Institutions
In this quick activity, learners observe how the added sugar in a can of soda affects its density and thus, its ability to float in water.

Ocean Acidification in a Cup
Source Institutions
Ocean acidification is a problem that humans will have to deal with as we release more and more carbon dioxide into the atmosphere.

Acid Rain Effects
Learners conduct a simple experiment to model and explore the harmful effects of acid rain (vinegar) on living (green leaf and eggshell) and non-living (paper clip) objects.

What's Hiding in the Air?: Acid Rain Activity
As a model of acid rain, learners water plants with three different solutions: water only, vinegar only, vinegar-water mixture.

Radioactive Decay of Candium
Source Institutions
In this simulation, learners use M&M™ candy to explore radioactive isotope decay.

Composting
Source Institutions
In this environmental science activity, learners research what is essential for plant life and the necessary components of soil to support plants.
