Search Results
Showing results 1 to 20 of 463

Cleaning with Dirt
Source Institutions
Learners build a filter from old soda bottles and dirt. They create polluted water, and pour it through their filter to clean it.
Where is the Sun?
Source Institutions
In this activity, learners verify that the Sun appears in a different location at a specific time every day of the year with one exception: on the Equinoxes.

A Little Drop of Water: Cohesion
Source Institutions
Learners explore water's property of cohesion through two investigations.

Hot Stuff!: Investigation #4
Learners test two jars containing soil, one covered and one open, for changes in temperature. After placing the jars in the Sun, learners discover that the covered jar cools down more slowly.

Dripping Wet or Dry as a Bone?
Learners investigate the concept of humidity by using a dry and wet sponge as a model. They determine a model for 100% humidity, a sponge saturated with water.

Rusty Penny
Source Institutions
In this easy chemistry activity, learners submerge pennies in different liquids (water, lemon juice, vinegar, liquid hand soap, salt water, and baking soda mixed with water) to observe which best clea

Acid (and Base) Rainbows
Learners use red cabbage juice and pH indicator paper to test the acidity and basicity of household materials. The activity links this concept of acids and bases to acid rain and other pollutants.

Modeling Day and Night
Source Institutions
In this activity (on page 1 of the PDF), learners make a "mini-globe" to investigate the causes of day and night on our planet.

The Geophysical Light/Dark Cycle
Source Institutions
This is an activity (located on page 131 of the PDF) related to sleep and circadian rhythms as well as space travel.

Meteoroids and the Craters They Make
Source Institutions
In this activity, learners investigate the formation of craters. Learners will examine how the size, angle and speed of a meteorite's impact affects the properties of craters.

Hot Stuff!: Investigation #1
Learners test two jars, one containing plain air and one containing carbon dioxide gas, to see their reactions to temperature changes.

Avalanche
Source Institutions
In this geology activity, learners create a model using a mixture of salt and sand inside a CD case. When the case is tilted or inverted, the mixture dramatically sorts into a layered pattern.

From the Internet to Outer Space
Source Institutions
In this activity, learners will use Google Sky to observe features of the night sky and share their observations.
Mercury in the Environment
Source Institutions
In this environmental science lesson, learners will examine the dangers of mercury and how humans contribute to growing mercury emissions on Earth.

A Funny Taste
Source Institutions
In this activity, learners explore the different salinities of various sources of water by taste-testing.

How Much Water is in that Cloud?
Source Institutions
In this activity, learners working in pairs saturate a cotton ball using water drops from an eyedropper to demonstrate the high water capacity of clouds.
Amazing Albedo
Source Institutions
In this experiment, learners work in teams to investigate how the color of a surface influences its ability to reflect light and therefore heat.
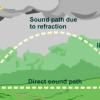
The Rumblin' Road: Determining distance to a Thunderstorm
Source Institutions
In this activity, learners discover how to determine the distance to a lightning strike or nearby thunderstorm.

The Power of Words
Source Institutions
This simple, yet surprising physics demonstration challenges preconceptions about forces, and demonstrates the strength of atmospheric pressure.
Blowin’ Up a Storm of Oil
Source Institutions
In this activity, learners investigate how wind can create surface currents and how waves move. Learners also discover how wind can affect oil spills.
