Search Results
Showing results 41 to 60 of 114
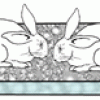
Acid Rain Eats Stone!
Source Institutions
This display shows the dangers of acid rain on buildings and other structures as two concrete bunny rabbits are disintegrated by sulfuric acid. Learners scrape chalk onto the concrete bunnies.

Raising the Level of Carbon Dioxide in Your Blood
Source Institutions
In this activity (on page 146 of the PDF), learners will explore the effects of increased carbon dioxide in the bloodstream.

Hot Stuff!: Creating and Testing for Carbon Dioxide
In this demonstration, learners observe vinegar and baking soda reacting to form carbon dioxide (CO2) gas.

Yeast-Air Balloons
Source Institutions
In this activity, learners make a yeast-air balloon to get a better idea of what yeast can do. Learners discover that the purpose of leaveners like yeast is to produce the gas that makes bread rise.

Hot Stuff!: Investigation #1
Learners test two jars, one containing plain air and one containing carbon dioxide gas, to see their reactions to temperature changes.

Fuel for Living Things
Source Institutions
In this activity, learners observe what happens when yeast cells are provided with a source of food (sugar). Red cabbage "juice" will serve as an indicator for the presence of carbon dioxide.
What Molecules Make the Holes in Bread?
Source Institutions
In this activity, learners will discover why there are holes in bread.

Burning Issues
Source Institutions
Learners use a candle to investigate the products of combustion. When a glass rod is held over a lit candle, the candle flame deposits carbon on the rod.

Limewater
Source Institutions
This is a chemistry lab activity about solutions (page 6 of the PDF). Students make a limewater testing solution for carbon dioxide and explore the concepts of solubility and precipitates.

Atomic Mobile
Source Institutions
Learners make a mobile model of a carbon atom using clay, wire, and pipe cleaners.

Disappearing Statues
Source Institutions
In this activity (on page 8), learners model how marble statues and buildings are affected by acid rain.

Introduction to the Scientific Method
Source Institutions
In this activity (page 26 of the PDF), learners make observations, formulate hypotheses and design a controlled experiment, based on the reaction of carbon dioxide with calcium hydroxide.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Coffee to Carbon
Source Institutions
In this activity, learners place cards featuring biological structures in order by their relative size from largest to smallest.

Chemical Breath
Source Institutions
This is a chemistry lab activity about solutions (page 7 of the PDF). Learners see firsthand how chemicals in a solution can combine to form an entirely different substance.

Shell Shifts
Source Institutions
Ocean acidification is a big issue due to the amount of carbon dioxide humans release. CO2 in the atmosphere is absorbed into the ocean thus changing its acidity.

What Goes Around Comes Around
Source Institutions
In this simulation activity, learners act as parts of the circulatory system and discover how it serves as a transport system for food/nutrients, oxygen, carbon dioxide and waste.
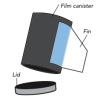
Pop Rockets
Source Institutions
In this activity, learners make film canister rocket ships. A fin pattern is glued onto the outside of the canister, and fuel (water and half an antacid tablet) is mixed inside the canister.

Dancing Spaghetti
Source Institutions
In this chemistry activity, learners use spaghetti to explore density and chemical reactions.
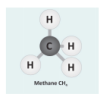
Let's Make Molecules
Source Institutions
In this activity, learners use gumdrops and toothpicks to model the composition and molecular structure of three greenhouse gases: carbon dioxide (CO2), water vapor (H2O) and methane (CH4).
