Search Results
Showing results 1 to 11 of 11

Bridge Building
Source Institutions
This is a quick activity (on page 2 of the PDF under Hockey Sticks Activity) about how the arrangement of carbon atoms determines carbon's different properties.

The Carbon Cycle and its Role in Climate Change: Activity 1
Source Institutions
In this activity (on page 1), learners role play as atoms to explore how atoms can be rearranged to make different materials.
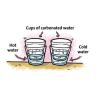
Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Raising the Level of Carbon Dioxide in Your Blood
Source Institutions
In this activity (on page 146 of the PDF), learners will explore the effects of increased carbon dioxide in the bloodstream.

Hot Stuff!: Investigation #1
Learners test two jars, one containing plain air and one containing carbon dioxide gas, to see their reactions to temperature changes.

It's a Gas, Man
Source Institutions
In this activity, learners discover if carbon dioxide has an effect on temperature.

How Long Can You Hold Your Breath?
Source Institutions
In this activity (on page 142 of the PDF), learners will compare breathing rates before and after hyperventilation to explore how reduced carbon dioxide levels in the blood lower the need to breathe.

Weight For It!
Source Institutions
In this activity about weights and balances, learners create their own balance using paper cups. Then, learners explore how to compare the relative mass of objects.

Hot Stuff!: Investigation #4
Learners test two jars containing soil, one covered and one open, for changes in temperature. After placing the jars in the Sun, learners discover that the covered jar cools down more slowly.

Hot Stuff!: Investigation #2
Learners test two jars containing hot water, one covered with plastic and one open, for changes in temperature.

Hot Stuff!: Investigation #3
Learners test two jars of ice water, one covered and one open, for changes in temperature. After placing the jars in the sun, learners discover that the covered jar cools down more slowly.
