Search Results
Showing results 1 to 20 of 29

Capturing Carbon Dioxide
Source Institutions
In this activity, learners investigate carbon sequestration by creating a carbonated beverage out of apple juice and dry ice.

Light Soda
Source Institutions
In this activity, learners sublimate dry ice and then taste the carbon dioxide gas.
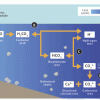
Coral, Carbon Dioxide and Calcification
Source Institutions
In this group activity, learners act out key stages of the "ocean carbon cycle" (also known as the "carbonate buffer system") through motions, rearranging blocks and team tasks.

The Carbon Cycle: How It Works
Source Institutions
In this game, learners walk through an imaginary Carbon Cycle and explore the ways in which carbon is stored in reservoirs and the processes that transport the carbon atom from one location to another

Make Your Own Soda Pop
Source Institutions
In this chemistry activity (page 8 of the PDF), learners will identify the instances of physical change, chemical change, and solutions while making homemade soda pop.

Breathing Blue
Source Institutions
In this activity, learners test exhaled breath for carbon dioxide and learn how to use an indicator as a simple way to measure pH.

Bready Bubble Balloon
Source Institutions
Learners discover the bubble power of living cells in this multi-hour experiment with baker's yeast. Learners make a living yeast/water solution in a bottle, and add table sugar to feed the yeast.

Breathing Yeasties
Source Institutions
Does yeast breathe? Find out by watching how plastic bags filled with yeast, warm water and different amounts of sugar change over time.

Temperature Affects the Solubility of Gases
Source Institutions
In this activity, learners heat and cool carbonated water to find out whether temperature has an effect on how fast the dissolved gas leaves carbonated water.

Having a Gas with Cola
Source Institutions
In this activity, learners measure the amount of carbon dioxide in a carbonated drink.

Hot Stuff!: Testing for Carbon Dioxide from Our Own Breath
Learners blow into balloons and collect their breath--carbon dioxide gas (CO2). They then blow the CO2 from the balloon into a solution of acid-base indicator.

A Dissolving Challenge
Source Institutions
In this activity, learners add objects and substances to carbonated water to discover that added objects increase the rate at which dissolved gas comes out of solution.

Off Base
Source Institutions
In this activity, learners explore the factors that tend to resist changes in pH of the ocean and why the ocean is becoming more acidic.

Hot Stuff!: Creating and Testing for Carbon Dioxide
In this demonstration, learners observe vinegar and baking soda reacting to form carbon dioxide (CO2) gas.
What Molecules Make the Holes in Bread?
Source Institutions
In this activity, learners will discover why there are holes in bread.

Limewater
Source Institutions
This is a chemistry lab activity about solutions (page 6 of the PDF). Students make a limewater testing solution for carbon dioxide and explore the concepts of solubility and precipitates.

Disappearing Statues
Source Institutions
In this activity (on page 8), learners model how marble statues and buildings are affected by acid rain.

Introduction to the Scientific Method
Source Institutions
In this activity (page 26 of the PDF), learners make observations, formulate hypotheses and design a controlled experiment, based on the reaction of carbon dioxide with calcium hydroxide.

"Boyle-ing" Water
Source Institutions
In this activity, learners explore Boyle's Law and discover that water will boil at room temperature if its pressure is lowered.

Chemical Breath
Source Institutions
This is a chemistry lab activity about solutions (page 7 of the PDF). Learners see firsthand how chemicals in a solution can combine to form an entirely different substance.
