Search Results
Showing results 1 to 20 of 30

Burn a Peanut
Source Institutions
In this activity, learners burn a peanut, which produces a flame that can be used to boil away water and count the calories contained in the peanut.

Pickle-oh!: Musical Pickle Instrument
Source Institutions
What's a Pickle-Oh? Two pieces of pickle on a stick are connected to a Pico Cricket (micro controller). When you slide the pickles apart the note changes.

Pie-Pan Convection
Source Institutions
It's difficult to see convection currents in any liquid that's undergoing a temperature change, but in this Exploratorium Science Snack, you can see the currents with the help of food coloring.

Marshmallow Puff Tube
Source Institutions
In this demonstration/activity, learners observe as a regular size marshmallow is blown through a tube made from a manila file folder.

Gumdrop Chains and Shrinky Necklaces
Source Institutions
In this activity, learners thread gumdrops together to make a model of a polymer.

Take an Egg for a Spin
Source Institutions
This is an activity about friction as well as kinetic and potential energy.

Cabbage Patch Chemistry
Source Institutions
In this chemistry activity, learners will learn how to make their own pH indicator using cabbage leaves, and then test common household items with their homemade indicator.

Experimenting with Naked Eggs
Source Institutions
In this activity about osmosis, learners use a naked egg (one with a dissolved eggshell) to learn about selectively permeable membranes.

Geyser
Source Institutions
This Exploratorium activity can be used in many contexts because geysers are great opportunities for learning about heat and temperature changes as well as geological/space science phenomena.

Gelatin Prism
Source Institutions
In this activity, learners make prisms from gelatin. Learners then shine light through the prisms and discover what happens. This activity introduces learners to the idea of refraction.

Hot & Cold
Source Institutions
In this activity, learners experiment with hydrogen peroxide, vinegar, yeast, and baking soda to produce hot and cold reactions. Use this activity to demonstrate exothermic and endothermic reactions.

Nano Ice Cream
Source Institutions
In this activity/demo, learners discover how liquid nitrogen cools a creamy mixture at such a rapid rate that it precipitates super fine grained (nano) ice cream.

Get the Porridge Just Right
Source Institutions
Learners set up three different bowls, each with a different mass of oatmeal. Learners monitor the temperature of the oatmeal and find that larger masses take longer to cool.

Freezing Lakes
Source Institutions
In some parts of the world, lakes freeze during winter. In this activity learners will explore water’s unique properties of freezing and melting, and how these relate to density and temperature.

Making Naked Eggs: Eggs Without Shells
Source Institutions
This is an activity about acid-base reactions using eggs and vinegar. Learners place eggs inside a container of vinegar and leave to soak overnight.

Mighty Molecules
Source Institutions
In this activity, learners use marshmallows and gum drops to construct seven models of molecules. Learners classify (solid, liquid or gas) and draw diagrams of the molecules.

Liquid Body Armor
Source Institutions
In this activity, learners explore how nanotechnology is being used to create new types of protective fabrics.
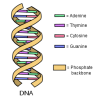
Close, Closer, Closest
Source Institutions
In this activity, learners perform an experiment that models a chromatography-like process called electrophoresis, a process used to analyze DNA.

Ziptop Bag Chemistry
Source Institutions
In this chemistry activity, learners perform three chemical reactions in a sealed zip-top bag. Learners will record their observations and classify the changes as chemical or physical.

Mixtures and Solutions
Source Institutions
This activity was designed for blind learners, but all types of learners can use it to investigate heterogeneous and homogeneous mixtures and solutions, identify the differences, and explore the conce
