Search Results
Showing results 1 to 20 of 50

Iodine Investigators!
Source Institutions
In this activity on page 7 of the PDF (Chemistry—It’s Elemental), learners use iodine to identify foods that contain starch.

To Dye For
Source Institutions
Learners add two dyes to mineral oil and water, and then compare their miscibility (how well they mix) in each.

Smell the Maillard Reaction
Source Institutions
In this activity, learners cook amino acids and sugar to explore the range of aromas released.

Hollandaise Sauce: Emulsion at Work
Source Institutions
In this activity, learners follow a recipe to make hollandaise sauce. Learners discover how cooks use egg yolks to blend oil and water together into a smooth mix.

Dunking the Planets
Source Institutions
In this demonstration, learners compare the relative sizes and masses of scale models of the planets as represented by fruits and other foods.

Mixing and Unmixing in the Kitchen
Source Institutions
In this chemistry investigation, learners combine common cooking substances (flour, baking powder, sugar, salt, pepper, oil, water, food coloring) to explore mixtures.

Gumdrop Chains and Shrinky Necklaces
Source Institutions
In this activity, learners thread gumdrops together to make a model of a polymer.
Growing Rock Candy
Source Institutions
In this activity, learners make their own rock candy. Crystals will grow from a piece of string hanging in a cup of sugar water. The edible crystals may take up to a week to form.

Toast a Mole!
Source Institutions
In this quick activity, learners drink Avogadro's number worth of molecules - 6.02x10^23 molecules!
Triboluminescence
Source Institutions
In this activity, learners discover what happens when they crush wintergreen-flavored candies in a very dark room.

Crunch and Munch Lab
Source Institutions
In this activity, learners use three types of cheesy snacks--cheese balls, cheese puffs, and Cheetos--to learn about polymers.
The Nose Knows!
Source Institutions
In this activity on page 9 of the PDF, learners test how flavoring extracts move through the walls of a balloon.

Diffusion of Water with Gummy Bears
Source Institutions
In this activity, learners investigate the movement of water into and out of a polymer. Learners test the diffusion of water through gummy bears, which are made of sugar and gelatin (a polymer).
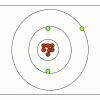
M&M® Model of the Atom: Edible Subatomic Particles
Source Institutions
In this activity, learners use colored candy to represent subatomic particles and make a model of an atom (Bohr model).

Inverted Bottles
Source Institutions
In this activity, learners investigate convection by using food coloring and water of different temperatures.

Sweetly Balanced Equations
Source Institutions
In this (edible) activity, learners balance chemical equations using different kinds and colors of candy that represent different atoms. Learners will work in pairs and explore conservation of atoms.

Avi's Sensational Salt Dough
Source Institutions
In this activity on page 5 of the PDF, learners mimic the process for making bricks. Learners shape and bake creations from a dough that is made from flour, salt, and water.

Mighty Molecules
Source Institutions
In this activity, learners use marshmallows and gum drops to construct seven models of molecules. Learners classify (solid, liquid or gas) and draw diagrams of the molecules.

A Slime By Any Other Name
Source Institutions
This fun video explains how to make a batch of oobleck (or slime) and why this special substance is known as a "non-Newtonian" fluid. Watch as Mr.

Oily Ice
Source Institutions
In this activity, learners experiment with the density of ice, water, and oil. Learners will discover that the density of a liquid determines whether it will float above or sink below another liquid.
